According to RealSelf, 96% of patients report high satisfaction with their Xeomin treatments, highlighting the confidence and positive results many individuals experience with this aesthetic option.
Xeomin, a botulinum toxin type A injectable, stands out for its highly purified formulation, which is free from additional proteins that could trigger adverse reactions. This unique feature minimizes side effects and ensures precise and consistent results when administered by trained medical professionals using the correct dosage and dilution.
This article provides a detailed look at the Xeomin reconstitution chart, required equipment and supplies, step-by-step instructions, dilution ratios, and clinical guidelines.
Key Takeaways
- Xeomin must undergo reconstitution, mixing the botulinum toxin type A formulation with a diluent to prepare it for injection.
- Medical professionals must have the equipment and supplies for each Xeomin reconstitution, such as sterile Xeomin vials, preservative-free saline, alcohol prep pads, and sterile syringes and needles.
- The Xeomin manufacturer has not recommended diluent options other than saline, as others may affect the potency and safety of the reconstituted product.
- Accurate measurement ensures correct Xeomin dosage and proper mixing techniques prevent foam formation and maintain the product’s efficacy.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Xeomin online, our dedicated sales agents can give you proper guidance. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Introduction to Xeomin Reconstitution
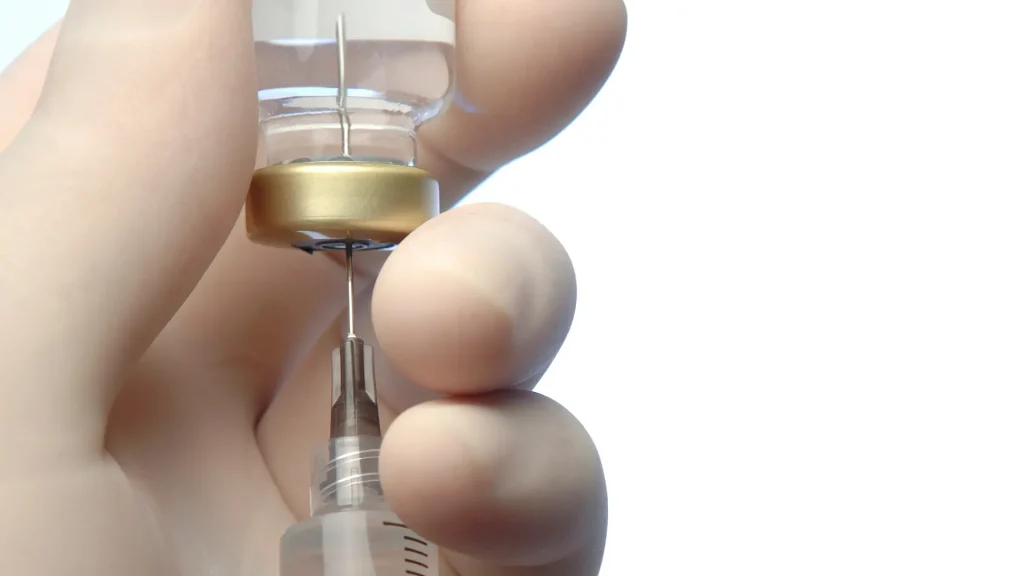
Xeomin (incobotulinumtoxinA) falls under the category of neuromodulator injection in medical aesthetics to temporarily enhance and smoothen moderate to severe upper facial lines, such as glabellar lines, forehead lines, and crow’s feet. When comparing Xeomin vs Jeuveau, it’s worth noting that these injections have received the US Food and Drug Administration approval.
Furthermore, Xeomin must undergo reconstitution before usage. This involves mixing the botulinum toxin type A formulation with a diluent to prepare it for injection. Medical professionals should understand that this process is critical to ensure correct dosage, patient safety, and treatment effectiveness.
Adherence to the Xeomin reconstitution chart is essential to maintaining the product’s safety and potency. Incorrect reconstitution leads to ineffective treatment, complications, or reactions that may compromise patient health and optimal outcomes.
Equipment and Supplies for Reconstitution
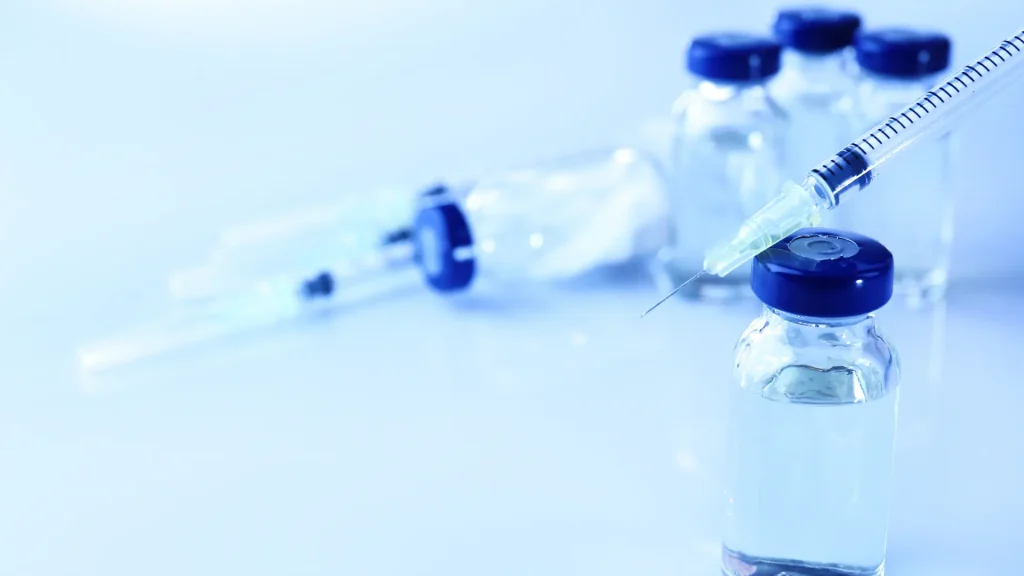
Before reconstitution, it’s essential to ensure that all the necessary things are prepared. Medical professionals must have the following equipment and supplies for each Xeomin reconstitution.
- Sterile Vial of Xeomin
- Preservative-Free Saline
- Alcohol Prep Pads
- Sterile Syringes
- Sterile Needles
Understanding the importance of using sterile and appropriate materials for Xeomin reconstitution significantly helps prevent contamination. This also ensures the effectiveness of the reconstituted product. Moreover, sterility sustains the product’s safety, and using the correct equipment ensures accurate dosing and treatment effectiveness.
Recommended Diluents for Xeomin
The most recommended and primary diluent for Xeomin reconstitution is a preservative-free 0.9% sodium chloride or saline solution. The manufacturer prefers this solution for its compatibility and safety profile.
The Xeomin manufacturer has not recommended any other diluent options, as others may affect the potency and safety of the reconstituted product. The correct diluent can help achieve optimal outcomes and maintain the product’s safety and efficacy.
Step-by-Step Reconstitution Process
Medical professionals must adhere to the proper reconstitution process. Xeomin has provided a step-by-step method for reconstituting the vial solution. Here are the following steps to ensure the Xeomin reconstitution.
- Vial Preparation: The exposed portion of the rubber stopper requires a thorough cleansing. Medical providers should use 70% alcohol before needle insertion.
- Saline Injection: The needle must be inserted vertically through the rubber stopper, where the vacuum will draw the saline into the vial. Gently inject the remaining saline into the vial to prevent foam prevention. If the vacuum does not pull the saline into the vial, ensure to discard Xeomin.
- Mixing: After removing the syringe, mix Xeomin with saline by swirling and flipping the vial. Avoid vigorous shaking of the vial.
Reconstituted Xeomin is a clear, colorless solution free of particulate matter. Accurate measurement ensures correct Xeomin dosage and proper mixing techniques prevent foam formation and maintain the product’s efficacy. Following these steps is crucial to delivering safe and effective treatment.
Dilution Ratios for Different Treatment Purposes
There are various dilution ratios for various treatment areas. Xeomin has provided a table that shows the diluent volumes for Xeomin reconstitution. For glabellar lines, the typical dosage is 20 units, requiring 0.25 mL of 0.9% saline solution in a 50-unit vial for reconstitution. Here are the other dilution ratios for different units.
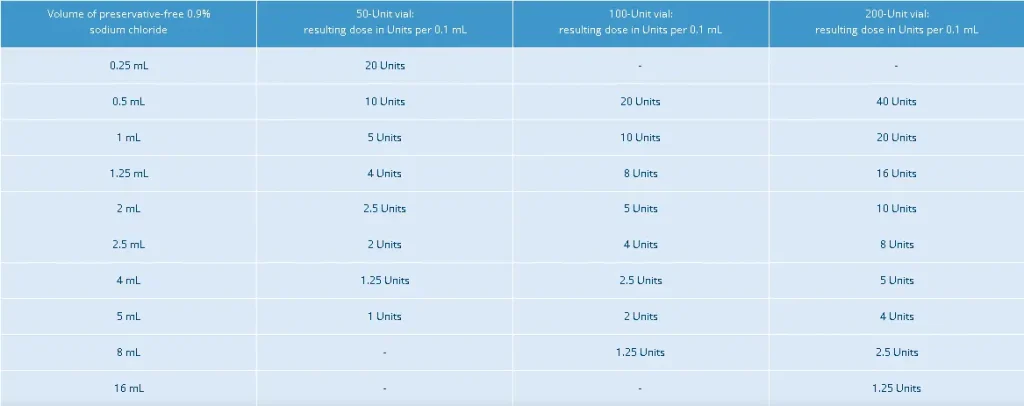
They have provided the specific units and injection sites for the dosing and administration of Xeomin for its approved therapeutic indications. The dilution ratios may also vary depending on the specific goals and patient needs. Medical professionals may use higher concentrations for more severe conditions, while lower ones are for aesthetic purposes.
Ensuring Sterility and Safety
Medical professionals must understand the importance of maintaining sterility in ensuring product and patient safety. To maintain sterility during Xeomin reconstitution, employ sterile gloves, disinfect the vial stopper with alcohol, and utilize sterile syringes and needles. Refrain from touching the needle or vial stopper post-disinfection.
Proper handling and storage of reconstituted Xeomin are essential to preserve its efficacy and safety. The reconstituted solution should be stored in a refrigerator at 2 to 8°C and utilized within 24 hours. Any unused solution must be discarded after this period. Moreover, each vial should be utilized for a single injection session and administered to only one patient.
Clinical Guidelines and Expert Recommendations
The clinical guidelines for Xeomin reconstitution involve using the primary component of preservative-free 0.9% saline solution and maintaining sterility throughout the process. Adherence to the proper Xeomin reconstitution is the expert recommendation that medical professionals should follow before administering the injection.
Properly cleanse the rubber stopper using 70% alcohol. Insert the 20—to 27-gauge needle through the rubber stopper, permitting the vacuum within the Xeomin vial to draw the saline from the syringe into the vial. After removing the needle, mix the contents by swirling and flipping.
Reconstituted Xeomin that is not used immediately must be stored in its original vial and refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours until the time of use. Following established protocols is crucial for the safety and effectiveness of Xeomin treatments. Correct reconstitution, handling, and storage practices for reliable and successful treatment outcomes.
Conclusion
The Xeomin reconstitution chart plays a critical role in ensuring the safety, efficacy, and optimal outcomes of Xeomin treatments. By following the recommended reconstitution process, medical professionals can maintain the product’s potency and deliver precise dosages, minimizing the risk of adverse reactions and treatment complications.
The meticulous approach outlined in the Xeomin reconstitution chart reflects a commitment to maintaining the highest standards in medical aesthetics. The emphasis on using sterile equipment, adhering to specific dilution ratios, and employing proper mixing techniques underscores the dedication to patient safety and treatment effectiveness in aesthetic enhancements.
FAQs
1. Why is reconstitution necessary for Xeomin?
Reconstitution is necessary to prepare the botulinum toxin type A formulation with a diluent for injection, ensuring accurate dosage, patient safety, and treatment effectiveness.
2. What equipment and supplies are essential for Xeomin reconstitution?
Medical professionals must have sterile Xeomin vials, preservative-free saline, alcohol prep pads, sterile syringes, and needles for each reconstitution to ensure proper mixing and accurate dosing.
3. What is the recommended diluent for Xeomin reconstitution?
The primary and recommended diluent for Xeomin reconstitution is a preservative-free 0.9% sodium chloride or saline solution, as other diluents may affect the safety and potency of the reconstituted product.
References
- Xeomin Reviews. (n.d.). RealSelf. Retrieved August 22, 2024, from https://www.realself.com/reviews/xeomin
- XEOMIN® Dosing & Administration Guide – Complete & Thorough. (2024, April 15). Xeomin. https://hcp.xeomin.com/healthcare-professionals/administering-xeomin/#









