In today’s healthcare landscape, patient feedback and online reviews play an increasingly important role in shaping treatment decisions and care delivery. According to the Harvard Business Review, patient experience has become a strategic priority, with continuous feedback helping to improve service design, adherence, and outcomes.
When it comes to therapies like Xolair (omalizumab), a biologic treatment for allergic asthma, chronic urticaria, and nasal polyps, understanding both patient and practitioner perspectives is key. Real-world patient reviews provide valuable insights into tolerability, convenience, and perceived effectiveness, while clinician observations help contextualize these experiences with clinical trial data and safety monitoring. This combined approach enriches shared decision-making, empowering patients while supporting professional judgment.
In this article, we will dive into patient reviews and clinician insights on Xolair, discussing its reported benefits and drawbacks, highlighting safety signals from clinical practice, and offering practical takeaways for both healthcare providers and patients.
Key Takeaways
- Xolair (omalizumab) is FDA-approved for allergic asthma, chronic spontaneous urticaria (CSU), chronic rhinosinusitis with nasal polyps (CRSwNP), and food allergies, with real-world feedback supporting its effectiveness in these conditions.
- Patient reviews highlight Xolair’s rapid onset of action, especially in conditions like CSU, where some people report noticeable improvement within 2 days of the first injection.
- Xolair has shown significant benefits in managing asthma and improving the quality of life for long-term sufferers, with patients noting better breathing and fewer exacerbations.
- Healthcare providers face challenges in administering Xolair, such as patient education for self-injection, administrative delays, and the need for post-injection monitoring due to the anaphylaxis risk.
- Ensuring patient adherence to the 2-4 week dosing schedule and providing ongoing support through structured follow-ups helps optimize Xolair’s effectiveness in long-term therapy.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Xolair, contact Medica Depot’s sales representatives and they will guide you on how to do so. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Real-World Effectiveness Of Xolair In Asthma, Urticaria, And Food Allergy From Patient Reports
Xolair (omalizumab) has proven to be a powerful therapy for managing allergy-related conditions, including asthma, chronic spontaneous urticaria (CSU), chronic rhinosinusitis with nasal polyps (CRSwNP), and food allergies. The FDA has approved Xolair for these conditions, offering patients and healthcare providers a reliable option when standard treatments fall short.
For many patients, Xolair has significantly improved symptom control, with real-world feedback shedding light on its effectiveness. While individual responses or Xolair reviews may vary, shared success stories provide insight into the benefits and risks of this biologic therapy. People planning to receive Xolair should read various reviews from treated patients.
One patient, who struggled with CSU, shared a five-star rating for Xolair, noting that despite no improvement from antihistamines, the injectable had a rapid onset of action, with visible effects just two days after the first injection. This represents an unusually fast response compared to typical clinical trial data, which shows improvement over 4–12 weeks, with mean remission times around 9.3 weeks.
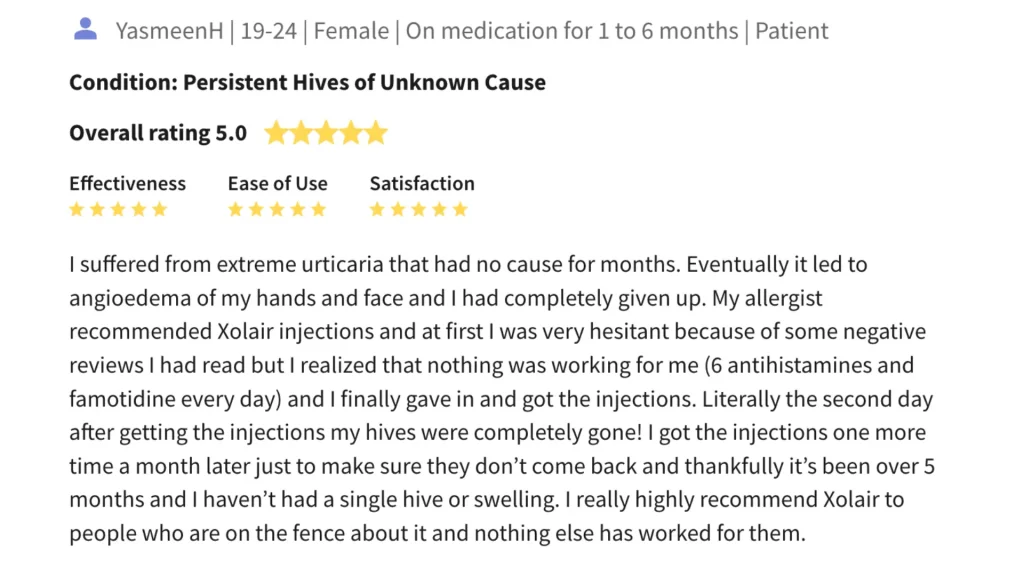
A patient with long-term allergic asthma reported a significant improvement in both breathing and overall quality of life after starting Xolair treatment. These outcomes align with Xolair’s demonstrated ability to reduce exacerbations and improve respiratory function.

Furthermore, patients with food allergies have also seen positive results. One individual reported a marked reduction in allergic reactions to cooking fumes from foods they were allergic to. The patient experienced minimal side effects and was able to avoid anaphylaxis, a significant concern for food allergy sufferers.
Note: When considering Xolair and pregnancy, patients may wonder if this treatment is safe. Current registry data shows no clear connection between Xolair and major birth defects. However, Xolair still needs further assessment to understand its potential reactions or side effects during pregnancy.
Practitioner Perspectives On Xolair’s Benefits, Challenges, And Clinical Workflow Integration
In clinical practice, healthcare providers observe clear benefits of Xolair in treating patients with allergic asthma, CSU, and food allergies. In trials, Xolair demonstrated reductions in asthma exacerbations, rapid symptom relief in CSU, and measurable improvements in quality of life for people with allergy-related conditions. These outcomes continue in routine clinical settings.
The predictable dosing algorithms and multiple subcutaneous delivery options (including prefilled syringes, vials, and autoinjectors) ease clinic workflows and help improve patient adherence. With the Xolair prescribing information, providers have all the necessary guidance from pre-treatment to post-treatment, including boxed warnings, dosing tables, and administration guidance.
Despite these advantages, real-world use of Xolair can present some challenges:
- Patient education and self-administration training take time, particularly when using an autoinjector or prefilled syringe.
- Administrative tasks, such as prior authorization, cold-chain logistics, and coordination with specialty pharmacies, can delay therapy initiation.
- For asthma patients, IgE levels and weight must be assessed before determining the correct dose, which adds an additional step to the workflow.
- Post-injection monitoring and precautions for persistent hives or anaphylaxis add to the clinical responsibilities, as Xolair carries a boxed warning for severe hypersensitivity reactions.
Patient Adherence, Satisfaction, And Access Barriers In Long-Term Xolair Therapy
Long-term adherence to Xolair therapy is often high when people experience significant symptom improvement. The two- to four-week subcutaneous schedule requires regular clinic visits, which can be a barrier for some people. However, self-administration training can improve adherence by offering more convenience and flexibility.
Patient education materials and hands-on training with autoinjectors help improve patient confidence in their ability to manage treatment independently. Streamlining clinic visits with standardized protocols and dosing checklists can also reduce errors and enhance patient satisfaction.
Clear, open communication between healthcare providers and patients is essential to maintaining safe and effective long-term therapy. Practitioners should encourage structured follow-up with tools like symptom scores, exacerbation logs, and rescue medication tracking to track treatment effectiveness and guide future treatment decisions.
Lessons From Clinical Experience: Setting Expectations And Improving Treatment Outcomes
Setting realistic expectations is key to patient satisfaction and adherence. Providers should ensure people are well-informed about what to expect from Xolair therapy. They should have discussions about initial response timelines to potential side effects.
Healthcare providers should focus on:
- Explaining the approved indications for Xolair and how it fits the patient’s condition.
- Describing the typical response timeline. Note that while some people experience improvement within weeks, others may need months for maximal benefit.
- Emphasizing the importance of adhering to the prescribed 2–4-week subcutaneous schedule and reviewing the baseline tests required for asthma dosing.
- Reviewing safety precautions, including the boxed warning for anaphylaxis and the necessity for post-injection monitoring to ensure patients can promptly report any symptoms.
Conclusion
Xolair offers significant benefits for people managing allergic conditions like asthma and chronic spontaneous urticaria. Real-world feedback reveals that many people appreciate its rapid effects and improvements in quality of life. Both patients and healthcare providers benefit from sharing these experiences to guide treatment decisions.
However, challenges like patient training, administrative hurdles, and monitoring for side effects must be addressed. Ensuring patients are well-educated about self-administration and adherence can enhance treatment outcomes. Ultimately, combining patient experiences with clinical insights helps maximize the benefits of Xolair while managing any associated risks.
FAQs about the Xolair Reviews of Patients
1. What conditions does Xolair treat?
Xolai has FDA approval for the treatment of allergy-related conditions. These conditions are asthma, chronic spontaneous urticaria, food allergies, and chronic rhinosinusitis with nasal polyps.
2. How do patients feel about Xolair’s effectiveness?
Many people report positive experiences with Xolair. They note significant improvements in symptoms and quality of life, especially in breathing and allergic reactions.
3. What challenges do practitioners face when using Xolair?
Healthcare providers encounter challenges such as training people for self-injection, dealing with administrative tasks like prior authorization, and the need to monitor patients for potential side effects, including anaphylaxis.
References
Glaser J. 5 Principles to Improve the Patient Experience. Harvard Business Review. Published November 11, 2021. Accessed November 7, 2025. https://hbr.org/2021/11/5-principles-to-improve-the-patient-experience
Genentech Inc. XOLAIR® (Omalizumab) HIGHLIGHTS of PRESCRIBING INFORMATION. Genentech Inc. Accessed November 7, 2025. https://www.gene.com/download/pdf/xolair_prescribing.pdf