Did you know that nearly 48% of Food and Drug Administration (FDA) approvals pertain to medical devices? This statistic highlights the rapid expansion of the aesthetic and therapeutic medicine sector, which aims to improve appearance and well-being while prioritizing safety and efficacy.
The FDA plays a crucial role in assessing medical products, including drugs and devices, for their safety, efficacy, and reliability. Obtaining FDA approval indicates that a product has passed stringent testing to meet high standards. For instance, South Korea’s Neuramis injection has not yet received FDA approval for treating facial aging.
This article will delve into the FDA approval status of Neuramis fillers, the regulatory process, and the significance of safety evaluations and clinical trials to demonstrate their efficacy.
Key Takeaways
- Medical devices like dermal fillers should undergo extensive clinical trials and tests to prove their safety and effectiveness.
- The FDA approval is essential to ensure that medical devices meet the regulatory standards and stringent safety and efficacy requirements before reaching a broader market in the United States.
- Aesthetic professionals can use Neuramis fillers on lips, cheeks, chin, and jawline to target facial aging signs, but Neuramis fillers have yet to receive FDA approval in the United States.
- Clinical trial results suggest that Neuramis Deep is safe and effective for treating nasolabial folds.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Order Neuramis online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
The FDA Approval Process for Dermal Fillers

The US Food and Drug Administration (FDA) approval process involves a thorough evaluation of clinical data to ensure the safety and efficacy of drugs and medical devices, including dermal fillers. The key steps include:
- Device Discovery and Concept: Initial design and planning of the filler formulation.
- Pre-Clinical Research (Animal Testing): Assessing basic safety and effectiveness in animal models.
- Pathway to Approval (Human Testing): Conducting human clinical trials to determine how well the filler performs.
- FDA Review: Evaluating all clinical trial data to ensure compliance with safety standards.
- FDA Post-Market Safety Monitoring: Continuous monitoring once the filler is approved and in use to track any adverse effects.
Specific Requirements for Dermal Fillers
Dermal fillers must undergo extensive clinical trials to verify their ability to safely target visible signs of aging and provide facial rejuvenation. The FDA also reviews:
- Manufacturing Practices: Ensuring consistent and safe production processes.
- Labeling: Providing clear usage instructions and potential risks to consumers and healthcare providers.
- Ingredients and Technologies: Assessing the hyaluronic acid or other materials used and the cross-linking technologies involved in their formulation.
Receiving FDA approval is crucial for ensuring that dermal fillers meet stringent safety and efficacy standards before they are marketed. This approval guarantees the product has been rigorously tested, protecting patients from potential health risks associated with unapproved or poorly regulated products. It also helps build trust with medical professionals, giving them confidence in using FDA-approved fillers to achieve safe and satisfactory results.
Neuramis Fillers

Neuramis fillers were developed by the South Korean company Medytox to address facial aging, volume loss, and contouring needs. These injectables use hyaluronic acid combined with innovative SHAPE technology, involving a two-step cross-linking process to provide enhanced duration and high purity.
Since entering the global market, Neuramis has gained popularity in Europe and Asia for its effective results, including the application of Neuramis fillers on lips for enhanced volume and definition.
Current FDA Approval Status
Despite their popularity, Neuramis fillers have not yet received FDA approval in the United States. This limits their availability in certain regions and emphasizes the importance of regulatory acceptance to expand their market reach.
Achieving FDA approval is crucial for Neuramis fillers, as it validates their safety and efficacy in treating facial skin concerns. FDA approval would not only boost confidence among practitioners and patients but also enable broader use across different markets, contributing to increased global accessibility and trust in the product’s reliability.
Safety and Efficacy of Neuramis Fillers
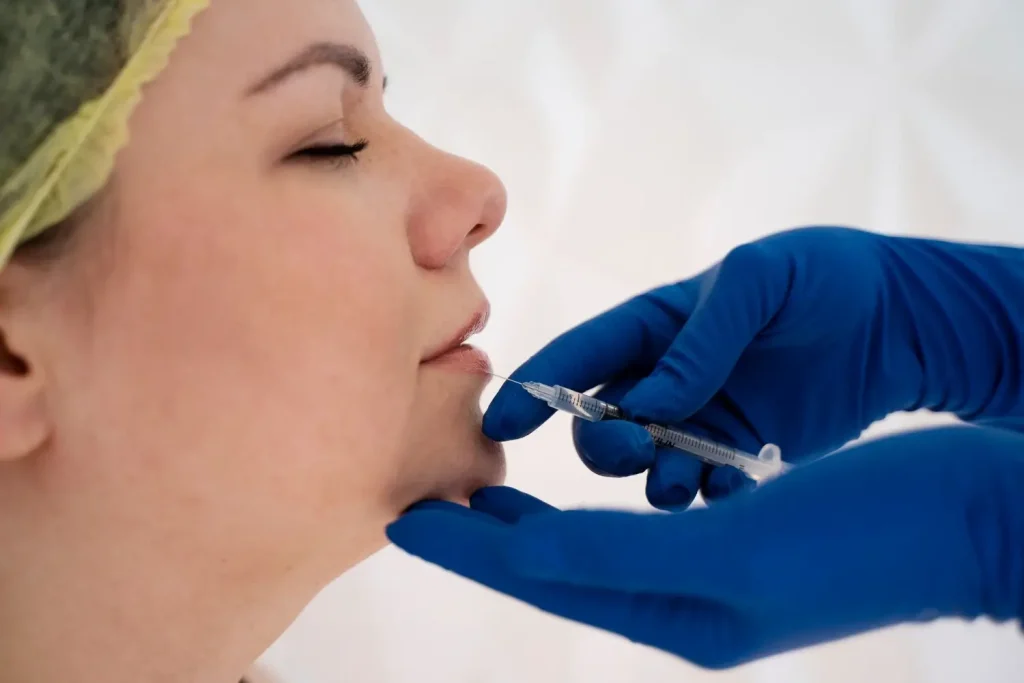
In a study by Pak et al. (2015), Neuramis Deep demonstrated comparable effectiveness to Restylane in improving nasolabial folds. The study found no significant differences in efficacy or safety between the two fillers. These results suggest that Neuramis Deep is safe and effective for treating nasolabial folds.
Furthermore, Neuramis and Perlane-L show similar efficacy and safety in improving nasolabial folds, as measured by the Wrinkle Severity Rating Scale and Global Aesthetic Improvement Scale. However, the difference in pain reduction between the two fillers is not clinically significant.
Despite Neuramis fillers’ proven safety and efficiency, individuals should expect common Neuramis side effects, particularly at the injection site, to still occur. These are often temporary and may subside within a few days to a week once the body adapts to the solution.
- Mild Swelling
- Redness
- Bruising
- Tenderness
Rare complications like allergic reactions and infections may occur if patients experience the wrong administration techniques. Additionally, the lack of Neuramis FDA approval affects the global marketing of dermal fillers, as these have yet to prove and reach the standard safety and efficacy in the United States.
Conclusion
The FDA approval process is essential for ensuring the safety and efficacy of medical devices, including dermal fillers like Neuramis. Although Neuramis fillers have not yet received FDA approval in the U.S., clinical trials have shown them to be safe and effective for treating nasolabial folds, similar to other FDA-approved fillers.
Achieving FDA approval will not only expand market access but also increase trust in the product’s reliability. Staying informed about regulatory status is key in the evolving field of aesthetic medicine.
FAQs
1. What is the significance of FDA approval for Neuramis fillers?
FDA approval ensures that Neuramis fillers have undergone rigorous testing to meet high safety and efficacy standards before being sold in the United States.
2. Where can Neuramis fillers be used, and are they FDA-approved in the United States?
Neuramis fillers can be used on lips, cheeks, chin, and jawline to target facial aging signs, but they have yet to receive FDA approval in the United States.
3. What clinical trial results suggest about the safety and effectiveness of Neuramis Deep?
Clinical trial results suggest that Neuramis Deep is safe and effective for treating nasolabial folds, demonstrating comparable effectiveness to other FDA-approved fillers.
References
- Myckatyn, T. (2018, February 27). What does an FDA approval mean for cosmetic treatments and devices? American Society of Plastic Surgeons. https://www.plasticsurgery.org/news/blog/what-does-an-fda-approval-mean-for-cosmetic-treatments-and-devices
- Joo, H. J., Woo, Y. J., Kim, J. E., Kim, B. J., & Kang, H. (2016). A Randomized Clinical Trial to Evaluate the Efficacy and Safety of Lidocaine-Containing Monophasic Hyaluronic Acid Filler for Nasolabial Folds. Plastic and reconstructive surgery, 137(3), 799–808. https://doi.org/10.1097/01.prs.0000479965.14775.f0