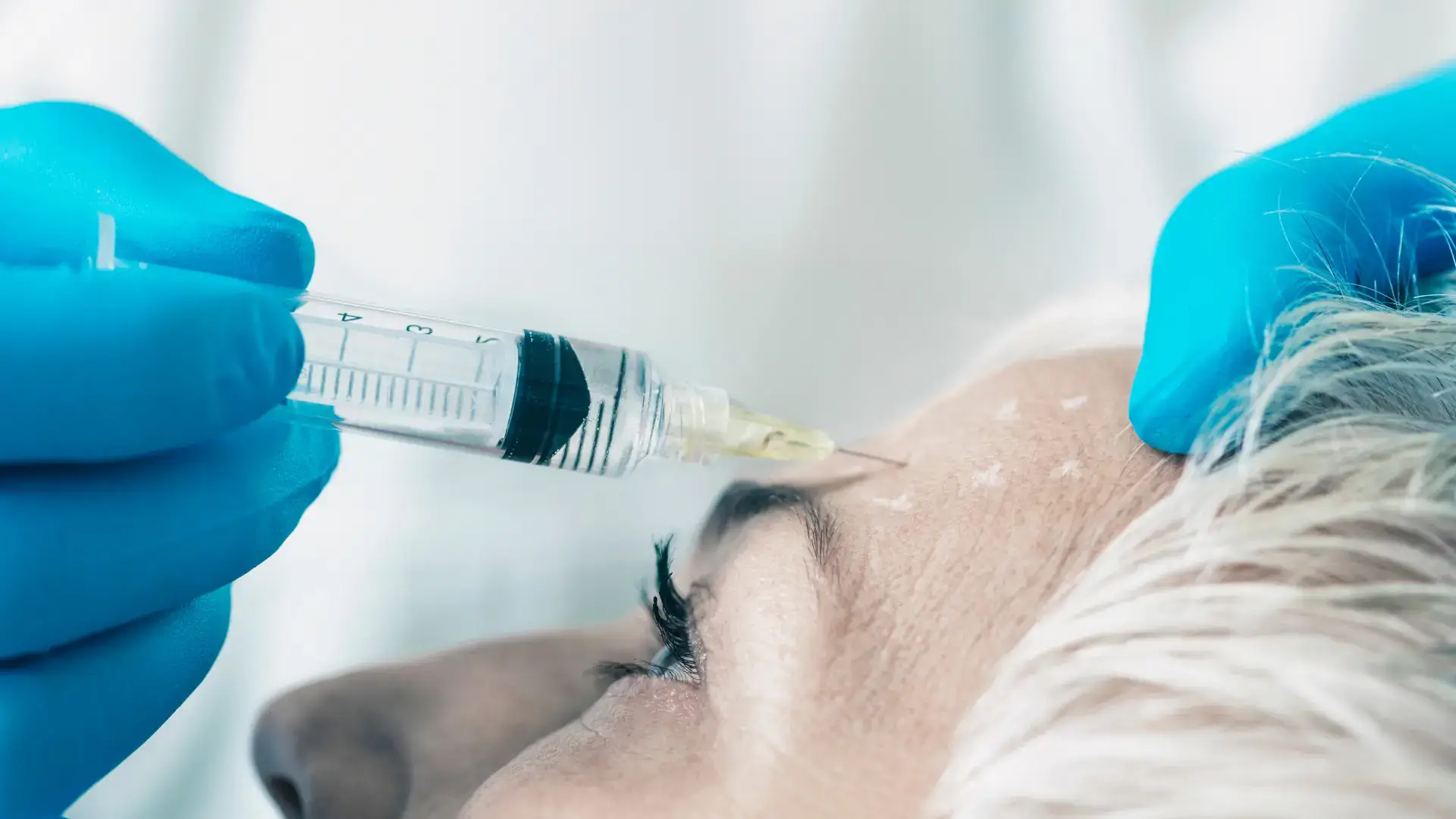
Minimally invasive procedures saw a 7% increase in 2023, surpassing traditional surgical options, according to the American Society of Plastic Surgeons. This trend highlights the growing demand for non-surgical treatments that offer effective results with less downtime.
Neuromodulator injections, such as Nabota and Dysport, have become the most popular choices for those seeking facial rejuvenation. Both offer unique botulinum toxin type A formulations, making them promising options for aesthetic enhancement.
In this article, we will compare Nabota vs Dysport, examining their compositions, approved uses, onset of action, duration of effects, and findings from clinical studies.
Key Takeaways
- Botulinum toxin type A injectables, in particular, are potent neuromodulators used for aesthetic and therapeutic applications.
- Despite their formula differences, Nabota and Dysport block the release of acetylcholine, halting muscle activity and resulting in relaxation.
- Medical professionals and individuals must understand the approved uses of these neuromodulators to ensure patient safety when administering these injections.
- Nabota requires 4 Units at each of the five injection sites, totaling 20 Units. In contrast, Dysport requires 10 Units at each of the five sites, resulting in 50 Units for glabellar lines.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Nabota online, our dedicated sales agents can give you proper guidance. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Introduction to Nabota and Dysport
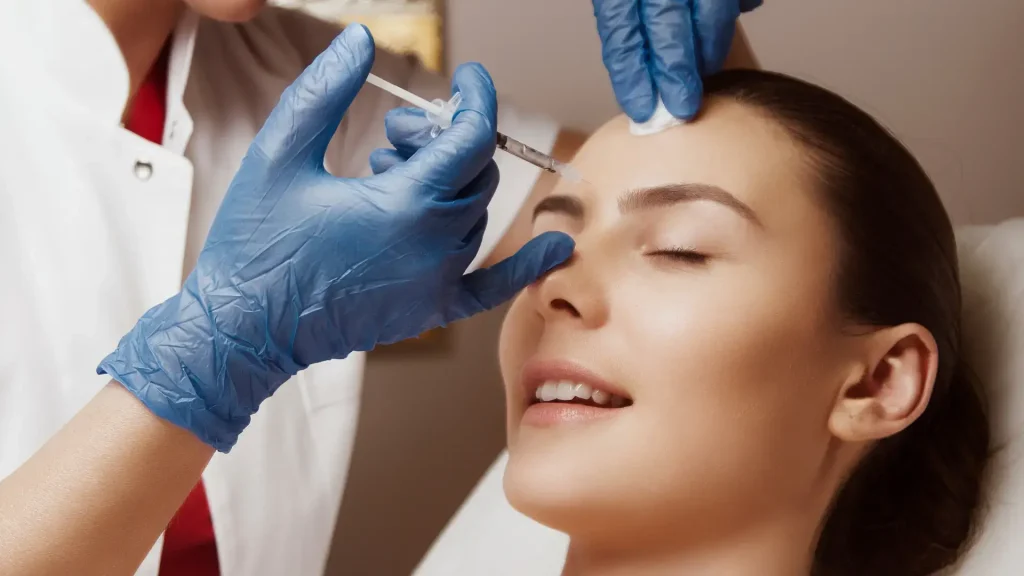
Botulinum toxin products derive their active ingredient from the bacterium Clostridium botulinum. Botulinum toxin type A injectables, in particular, are potent neuromodulators used for aesthetic and therapeutic applications. They weaken or paralyze skeletal muscles by inhibiting neurotransmission, resulting in muscle relaxation.
Initially utilized for medical conditions, botulinum toxin entered the aesthetic field as the top-sought minimally invasive treatment. While temporary, products like Nabota and Dysport effectively address facial aging signs.
However, when comparing neurotoxin injections like Nabota vs Xeomin or Nabota vs Dysport, patients must understand their similarities and differences. They should weigh factors such as cost considerations, onset of action, longevity of effects, preference, aesthetic concern, and goals.
Composition and Mechanism of Action
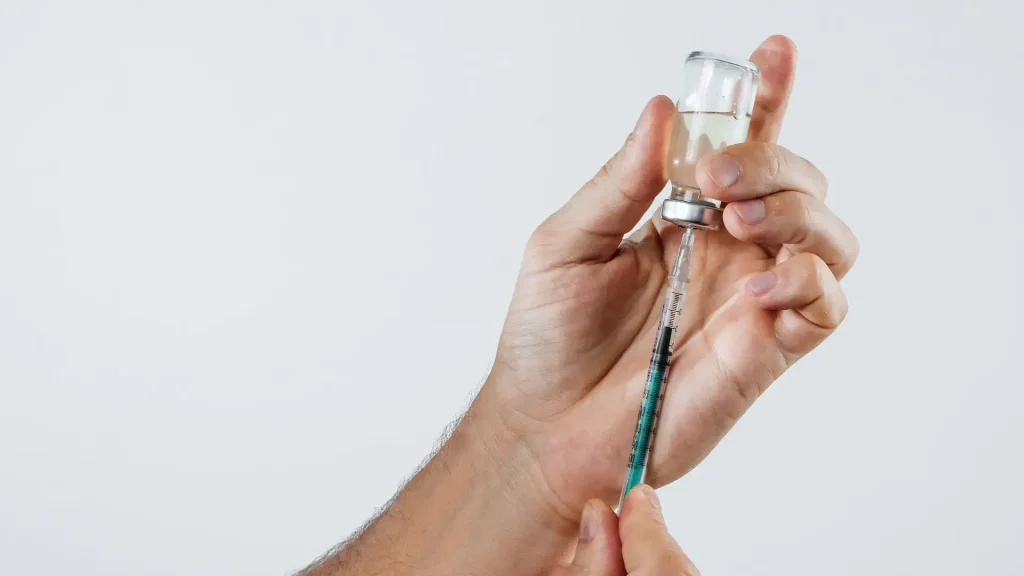
Individuals should know that neuromodulators utilize botulinum toxin type A in their injectables but may differ in formulations. Some injections use a highly purified botulinum toxin type A free from complex proteins, while others keep these accessory proteins in their formulations.
Daewoong Pharmaceutical developed Nabota in South Korea. It contains a highly purified botulinum toxin to minimize unnecessary proteins. They use a patented HI Pure Technology for its purity, leading to a faster onset of action and long-lasting effects.
Meanwhile, Dysport capitalizes on abobotulinumtoxinA, another type of botulinum toxin. This neuromodulator differs from Nabota with its formula, as it includes proteins and toxins. This helps in spreading further to other facial parts for a natural-looking result.
Despite their formula differences, Nabota and Dysport work similarly. When administered, they block the release of acetylcholine, a neurotransmitter causing muscle contractions. This action temporarily halts muscle activity, resulting in relaxation and improving the appearance of facial wrinkles and lines.
Indications and Approved Uses
Medical professionals and individuals must understand the approved uses of these neuromodulators to ensure patient safety when administering these injections. In particular, Nabota has received approval for use in different regions, highlighting its safety and efficacy.
- Glabellar Frown Lines: Approved by the US Food and Drug Administration in Europe, Canada, and South Korea
- Upper Limb Spasticity, Lateral Canthal Lines, and Blepharospasm: Approved in South Korea
On the other hand, Dysport also has specific indications for aesthetic and medical conditions. The US Food and Drug Administration approved these uses, emphasizing Dysport’s proven safety and effectiveness in addressing these concerns.
- Cervical Dystonia
- Glabellar Frown Lines
- Spasticity in Adults
- Upper Limb Spasticity in Pediatric Patients
- Lower Limb Spasticity in Pediatric Patients
It’s worth noting that despite these indications and approved uses of Nabota and Dysport, medical professionals may use these neuromodulators for off-label uses. However, this requires a more meticulous treatment process to ensure patients receive the maximum benefits while avoiding health compromises.
Onset of Action, Dosage Units, and Duration of Effects
When treating glabellar lines, medical professionals typically administer Nabota (Jeuveau) and Dysport at specific dosages. For Nabota, the recommended dose involves 4 Units at each of the five injection sites, totaling 20 Units. In contrast, Dysport requires 10 Units at each of the five sites, resulting in 50 Units for glabellar lines.
Dosages depend on the treatment area and the severity of the condition. Medical applications may require larger Units to treat these concerns. After injections, patients may want to know the onset of action and duration of the effects of these neuromodulators.
- Nabota’s Onset of Action and Longevity: Nabota is known for its swift onset of action, typically two days post-treatment. Patients can expect the effects to last within three to four months.
- Dysport’s Onset of Action and Longevity: Dysport may show noticeable effects within two to three days. Moreover, the effects may last longer than Nabota, three to five months.
Medical professionals may recommend maintenance or follow-up sessions for sustained results. Both neuromodulators have shown safety and potency in repeat treatments; however, patients must understand that individual factors may influence the results.
Patient Satisfaction and Clinical Studies

Patient satisfaction rates play a pivotal role in assessing the effectiveness and acceptability of treatments such as Nabota and Dysport. While Nabota does not have patient satisfaction online, its US FDA-approved brand, Jeuveau, has continued to satisfy cosmetic patients.
- Nabota: This neuromodulator boasts high patient satisfaction rates. A consumer survey of over 9,000 people treated with Jeuveau revealed that 94% were satisfied with their overall treatment experience. Moreover, RealSelf reviews for Jeuveau indicate an average ‘worth it’ rating of 91% from treated patients.
- Dysport: Clinical studies have consistently demonstrated high patient satisfaction with Dysport. In a study involving 120 individuals, an impressive 95% reported being highly satisfied with just two treatments per year. RealSelf shows that Dysport has an average ‘worth it’ rating of 94%.
Nabota and Dysport are both effective for aesthetic purposes. Nabota typically shows effects within two days, while Dysport may show effects within two to three days. These treatments target moderate to severe glabellar lines, significantly improving frown lines.
Medical professionals may also utilize these injectables for other facial aging signs, such as crow’s feet and smile lines. The results may last approximately three to five months, giving patients a more youthful appearance. Despite their effectiveness and favorable safety profile, Nabota and Dysport may cause common side effects.
- Nabota Side Effects: Injection site pain, swelling, redness, and bruising
- Dysport Side Effects: Injection site pain, injection site reaction, bruising, and swelling
These common side effects are temporary and typically resolve independently within a few days. Moreover, common adverse events may also occur to patients during rare occurrences, which include:
- Nabota: Headache, eyelid ptosis, upper respiratory tract infection, increased white blood cell count.
- Dysport: Nasopharyngitis, headache, injection site pain, injection site reaction, upper respiratory tract infection, eyelid edema, eyelid ptosis, sinusitis, nausea, and blood present in urine.
Conclusion
The comparison of Nabota vs Dysport highlights that both neuromodulators are effective options for facial and aesthetic enhancement. Understanding their approved uses, onset of action, dosage, and duration of effects is essential for ensuring patient safety and achieving the best results.
Nabota and Dysport offer distinct benefits, allowing healthcare professionals to tailor treatments to individual needs and preferences. Consulting with a qualified medical provider is crucial to selecting the most appropriate option based on specific aesthetic goals and medical considerations.
FAQs
1. What are Nabota and Dysport?
Nabota and Dysport are botulinum toxin type A injectables commonly used for facial or aesthetic enhancement options.
2. What are the similarities and differences between Nabota and Dysport?
Both Nabota and Dysport work similarly by blocking the release of acetylcholine, halting muscle activity, and resulting in muscle relaxation. Their differences lie in their compositions, approved uses, onset of action, and dosage units.
3. What are the approved uses of Nabota and Dysport?
Nabota is FDA-approved for glabellar frown lines, while Dysport is FDA-approved for cervical dystonia, glabellar frown lines, and spasticity in adults and pediatric patients.
References
- Plastic Surgery Sees Steady Growth Amidst Economic Uncertainty, American Society of Plastic Surgeons 2023 Procedural Statistics Report Finds. (2024, June 25). American Society of Plastic Surgeons. https://www.plasticsurgery.org/news/press-releases/plastic-surgery-sees-steady-growth-amidst-economic-uncertainty-american-society-of-plastic-surgeons-2023-procedural-statistics-report-finds
- Song, S., Lee, Y. H., Hong, J. P., & Oh, T. S. (2018). Safety, efficacy, and onset of a novel botulinum toxin type A (Nabota) for the treatment of glabellar frown lines: a single-arm, prospective, phase 4 clinical study. Archives of craniofacial surgery, 19(3), 168–174. https://doi.org/10.7181/acfs.2018.01886