The US Food and Drug Administration (FDA) plays a pivotal role in ensuring the safety and efficacy of treatments in the field of aesthetic medicine. For products like dermal fillers, including poly-L-lactic acid (PLLA), the FDA mandates rigorous clinical trials to demonstrate their long-lasting effects—often up to two years. This careful regulatory oversight helps maintain the high standards that consumers expect from cosmetic treatments.
As with all aesthetic enhancements, understanding whether a treatment is FDA-approved is crucial. Products like Lanluma, which aim to deliver skin rejuvenation and collagen stimulation, must meet stringent requirements to ensure their safety and effectiveness. For consumers, FDA approval offers peace of mind, knowing that the product has passed a thorough evaluation process.
In this article, we’ll explore whether Lanluma is FDA-approved, examine its benefits, applications, and compare it to other popular aesthetic treatments, helping you make an informed decision when considering this innovative option.
Key Takeaways
- Lanluma is a PLLA-based injectable that stimulates collagen production to restore volume and improve skin quality, with applications in both facial rejuvenation and body contouring.
- Lanluma is not FDA-approved for cosmetic use in the United States but holds a CE mark in Europe, which allows it to be legally used for aesthetic procedures across the European Economic Area.
- The FDA approval process in the U.S. involves rigorous clinical trials and safety evaluations, which Lanluma has not yet completed for aesthetic applications.
- Lanluma offers greater versatility compared to Sculptra, providing tailored formulations for both facial and body treatments. Meanwhile, Sculptra is FDA-approved primarily for facial rejuvenation.
- Practitioners must follow strict regulatory guidelines and clinical protocols when using Lanluma. This ensures patient safety and adherence to local regulations, especially in regions where it has not received FDA approval.
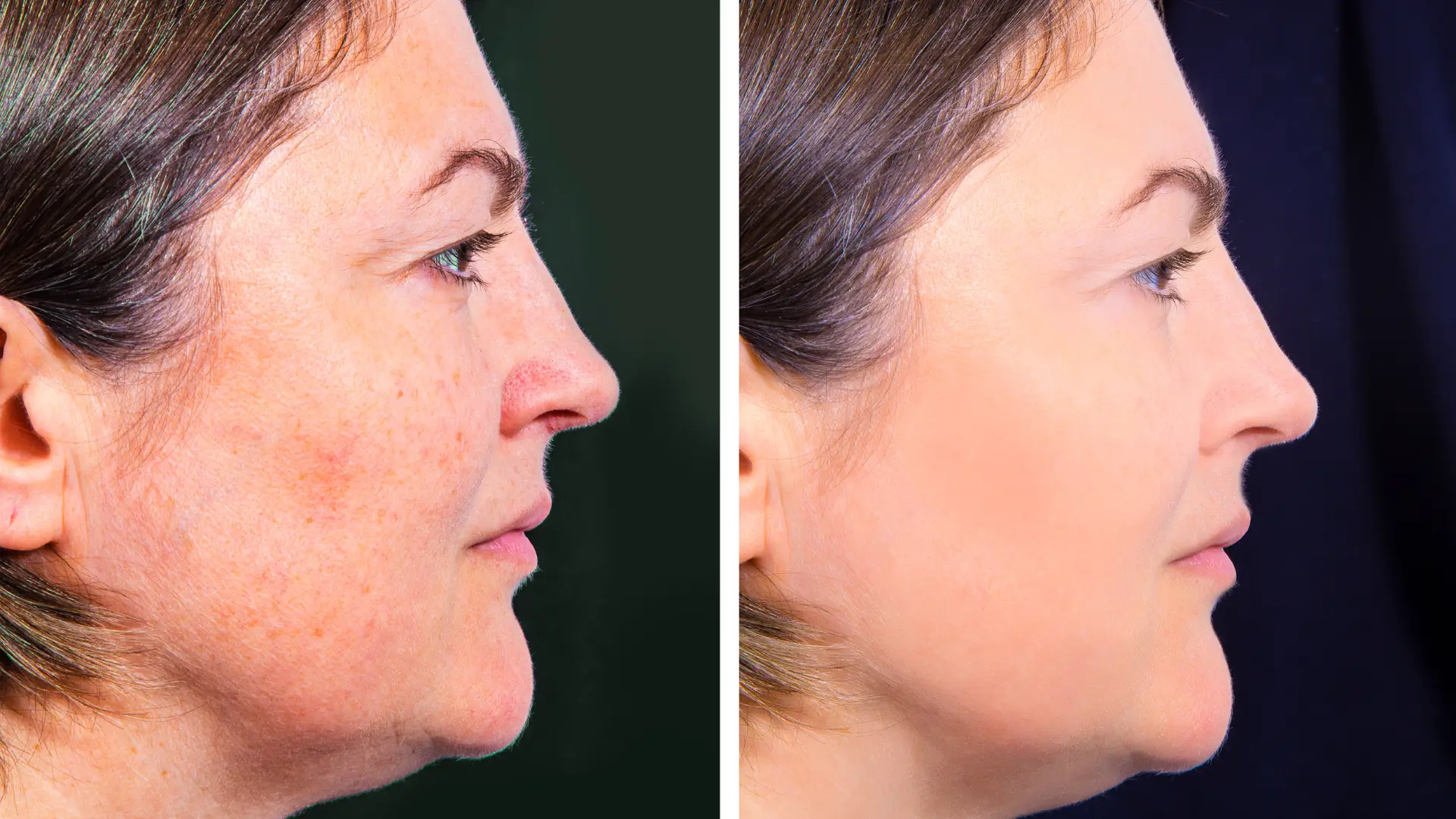
- Lanluma has shown promising results for collagen stimulation and volume restoration, with patients experiencing long-lasting and natural-looking results.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Lanluma Fillers online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Is Lanluma FDA Approved or CE Marked – What’s the Difference?

Individuals often prefer the convenience and safety of non-surgical aesthetic options, like poly-L-lactic acid (PLLA) injections. Among many choices in the market, many patients may ask their trusted practitioners, “What is Lanluma?” This PLLA-based injectable treatment works beneath the skin to stimulate collagen production, improving skin quality and volume over time.
However, its regulatory status differs by region, which practitioners should consider before utilizing the product. According to Sinclair’s official data and industry sources, Lanluma is not FDA-approved for cosmetic use in the United States, but it does hold a CE mark clearance in Europe. These differences arise from the varied regulatory requirements and evaluation processes in different parts of the world.
In the United States, obtaining FDA approval involves extensive clinical trials, rigorous safety, and efficacy evaluations, along with ongoing post-market surveillance. Since Lanluma has not yet met these stringent criteria for aesthetic applications, U.S. practitioners cannot legally use it for cosmetic purposes.
In contrast, the CE mark signifies that Lanluma complies with European health, safety, and environmental standards, allowing it to be marketed and used legally across the European Economic Area for cosmetic procedures.
Is Lanluma FDA Approved for Body Contouring or Just Facial Use?
Despite its lack of FDA approval in the United States, this PLLA-based injectable enhances collagen synthesis for tissue volumization and regeneration, providing longer-lasting effects compared to hyaluronic acid fillers or other minimally invasive options. Moreover, Lanluma boasts remarkable versatility for both facial rejuvenation and body contouring in regions where it has approval from regulatory bodies.
As it has received a CE mark in Europe, this approval authorizes Lanluma’s use for a range of applications, including facial and body areas, to increase volume in depressed regions and correct skin depressions.
In the United States, Lanluma has not received FDA approval for any aesthetic indication. U.S. practitioners must follow strict regulatory guidelines, meaning they cannot legally use products without FDA clearance, whether for facial enhancements or body contouring.
How Does Lanluma Compare to FDA-Approved Sculptra?
Both Lanluma and Sculptra provide PLLA-based injectables that stimulate collagen production to restore volume and enhance tissue quality. However, they differ in their formulation, regulatory applications, and clinical applications.
- Lanluma: Offers remarkable versatility with its V and X tailored formulations for both facial and body contouring.
- Sculptra: The FDA has approved it primarily for the treatment of facial lipoatrophy. Clinicians are increasingly using it off-label for body applications.
In clinical applications, Lanluma is versatile for both facial and body treatments, offering tailored dosing strategies. In comparison, clinicians primarily use Sculptra for facial rejuvenation, although they sometimes use it off-label for body contouring purposes.
What Practitioners Need to Know About Using Lanluma Legally

Practitioners must follow Sinclair’s official guidelines and all local regulatory requirements when using Lanluma. Manufacturers legally market this PLLA-based product only in regions where it has obtained the necessary clearances, such as a CE mark in Europe
Adhering to these legal protocols ensures patient safety and treatment efficacy while maintaining professional accountability. They should also familiarize themselves with the complete instructions for reconstituting the product, proper injection techniques, and necessary aftercare measures as specified by the manufacturer.
Practitioners must verify the regulatory status in their region. Also, keep in mind that the FDA has not approved Lanluma for cosmetic purposes in the United States. In every case, obtaining proper informed consent and maintaining detailed treatment records are critical steps to minimize legal risks.
Conclusion
While Lanluma shows promise as an effective PLLA-based injectable for enhancing aesthetics, it currently lacks FDA approval for cosmetic use in the United States. Its CE mark in Europe enables broader applications, including both facial and body treatments. This offers patients options for collagen stimulation and volume restoration.
For practitioners, it’s essential to stay informed about the regulatory landscape and the specific guidelines surrounding Lanluma’s use. Understanding its regulatory status and ensuring proper techniques, along with obtaining patient consent, can help avoid legal issues and enhance treatment outcomes, ultimately prioritizing patient safety and satisfaction.
FAQs
1. Does Lanluma have FDA approval for cosmetic use in the United States?
No, the FDA has not approved Lanluma for cosmetic use in the US. On the other hand, European regulatory authorities have granted it CE mark clearance for use in Europe.
2. What benefits does Lanluma provide as an injectable treatment?
Lanluma stimulates collagen production, which helps improve facial volume and skin texture, offering longer-lasting effects compared to traditional fillers.
3. Can practitioners use Lanluma for both facial and body treatments?
Yes, practitioners use Lanluma for both facial rejuvenation and body contouring, particularly in regions where regulatory authorities have approved its use.
References
- Center for Devices and Radiological Health. FDA-Approved Dermal Fillers. FDA. Published online November 4, 2021. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/fda-approved-dermal-fillers
- LANLUMA Revolutionary PLLA Collagen Stimulator. Lanluma. Accessed June 4, 2025. https://lanluma.com/healthcare-professionals/