According to Sullivan et al. (2022), physicians value clarity and specificity in FDA-approved Prescribing Information (PI). The study underscores PI’s critical role in enhancing drug communication between manufacturers and healthcare providers, ensuring that treatments are administered safely and effectively. Well-structured important safety information allows medical professionals to make informed decisions, ultimately improving patient care and treatment outcomes.
Understanding the prescribing guidelines is essential for medications like Cimzia. Proper dosing, administration, and safety precautions help optimize patient outcomes while minimizing risks. By familiarizing themselves with Cimzia’s PI, healthcare providers can ensure more effective treatment plans and improved patient satisfaction.
In this article, we will explore Cimzia’s prescribing information, including approved indications, dosing, administration guidelines, contraindications, pre-treatment testing, monitoring, and safety precautions.
Key Takeaways on Cimzia Important Safety Information
- Cimzia is an FDA-approved medication for various chronic inflammatory conditions in adults, including rheumatoid arthritis and Crohn’s disease, administered via subcutaneous injection.
- The recommended starter dose of Cimzia is 200 mg, given biweekly for the first four weeks, followed by tailored maintenance doses based on the treated condition.
- Proper administration for adults undergoing Cimzia therapy includes reconstitution of the lyophilized powder and injection techniques that minimize discomfort and ensure effectiveness.
- Key contraindications include severe hypersensitivity to the drug, concurrent use with live vaccines, and a lack of established safety data in pediatric patients.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Cimzia at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Overview of Cimzia’s Approved Indications

Cimzia (certolizumab pegol) is a prescription medication classified as a Tumor Necrosis Factor blocker. The US Food and Drug Administration has approved this minimally invasive solution to treat various conditions. The regulatory agency has also updated its label, highlighting its potential safety between Cimzia and pregnancy.
Cimzia treats several chronic inflammatory conditions in adults. According to the important safety information, conditions include:
- Moderate-to-severe rheumatoid arthritis
- Moderate-to-severe plaque psoriasis
- Moderate-to-severe Crohn’s disease
- Active psoriatic arthritis
- Active non-radiographic axial spondyloarthritis
- Active ankylosing spondylitis
Cimzia reduces inflammation and alleviates symptoms associated with these conditions. It is administered via subcutaneous injection and comes as either a lyophilized powder or a prefilled syringe. Moreover, this medication is not approved for use in children, and its safety and efficacy in pediatric patients have not been established.
Recommended Dose, Administration, and Contraindications
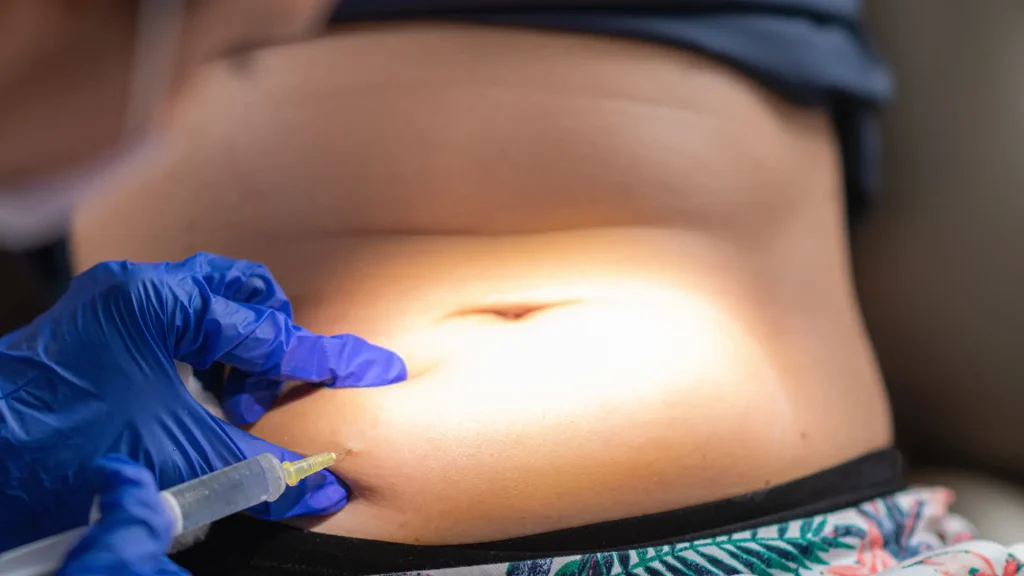
For optimal results, patients must begin with a starter dose before transitioning to the appropriate maintenance regimen based on their condition. The starter dosing consists of 200 mg injected twice every two weeks (at Weeks 0, 2, and 4) to establish therapeutic levels.
The maintenance dosing schedule varies by condition treated by Cimzia:
- Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, and Non-Radiographic Axial Spondyloarthritis: 200 mg every two weeks (14 days) or 400 mg (two 200 mg injections) every four weeks (28 days)
- Moderate-to-Severe Crohn’s Disease: 400 mg (two 200 mg injections) every four weeks (28 days)
- Moderate-to-Severe Plaque Psoriasis: 400 mg (two 200 mg injections) every other week, following the same schedule as the starter dose
Proper Administration
Medical professionals often use lyophilized powder for Cimzia injection, which requires appropriate reconstitution before injecting. Before administration, they should keep the reconstituted Cimzia at room temperature (not beyond two hours).
Practitioners can use a 20-gauge needle to withdraw the solution with the proper Cimzia dosage. They should replace it with a 23-gauge for administration, injecting the solution subcutaneously into the thigh or abdomen. Syringes should only be used once and replaced with a new one after every injection.
Furthermore, practitioners may also recommend self-injecting at home, wherein patients treated with Cimzia may utilize the prefilled syringe. They should let it warm to room temperature before use and discard the syringe if it is cloudy, discolored, or contains particulates.
Inject Cimzia into suitable sites such as the thigh or abdomen, ensuring the injection is at least 2 inches away from the navel. According to the important safety information, administer each injection at least 1 inch from the previous site.
Contraindications and Precautions
Additionally, practitioners and individuals should understand the product’s contraindications and precautions to avoid further complications. Avoiding the injection of Cimzia to patients with any contraindications can ensure patient safety and maximize its benefits.
- Severe hypersensitivity reaction to certolizumab pegol or any of the excipients
- Avoid administering Cimzia concurrently with live vaccines to prevent serious infections
- Not recommended for pediatric patients due to unestablished safety and efficacy
Pre-Treatment Testing and Monitoring
A thorough consultation with licensed and experienced medical professionals can help patients determine their suitability for treatment and ensure safety throughout the procedure. In addition to understanding the severity of the condition, the patient’s overall health, and concerns, practitioners should conduct specific pre-treatment tests to ensure patient safety before starting Cimzia treatment.
- Screen for Latent Tuberculosis (TB): If the test is positive, initiate appropriate TB treatment before starting Cimzia.
- Screen for Hepatitis B Virus (HBV) Infection: If reactivation of HBV occurs, patients should stop receiving TNF blockers like Cimzia and start with anti-viral therapy.
During Cimzia treatment, continuous monitoring is crucial to detect adverse effects or complications. This approach can help patients avoid further complications associated with the treatment procedure:
- Closely monitor patients treated for signs and symptoms of infection, including TB, throughout the treatment period.
- Conduct regular blood tests to monitor liver function and blood cell counts.
- Assess for any new or worsening neurological symptoms, as these may indicate the development of demyelinating disorders.
- Advise patients treated with Cimzia to promptly report any unusual symptoms or side effects to their healthcare provider.
Safety Precautions and Patient Care

To ensure the safe use of Cimzia, healthcare providers must educate patients about potential risks and advise them to report any unusual symptoms or side effects promptly. Important safety information on precautions includes:
- Adherence to the recommended treatment protocols, such as proper Cimzia dosing and administration.
- Regular follow-up to continuously monitor patients’ treatment condition, progress, and overall health.
- Monitor patients treated with Cimzia for signs of malignancies, including lymphoma and skin cancer, during and after treatment.
By adhering to these guidelines, healthcare providers can effectively manage and monitor patients to ensure the safe use of Cimzia. Furthermore, they should underscore the importance of adherence to the recommended post-treatment care instructions for a smooth recovery and longer duration of Cimzia effects.
Conclusion
Understanding the Cimzia prescribing information is essential for healthcare providers to ensure safe and effective treatment for patients suffering from chronic inflammatory conditions. They should adhere to the medication’s approved indications, dosing protocols, and appropriate administration techniques that can significantly enhance patient outcomes.
By following the important safety information, providers can optimize Cimzia’s therapeutic benefits while minimizing potential risks. Moreover, practitioners must stay informed about Cimzia contraindications and monitoring requirements to safeguard patient health. Like any medication, clear communication of possible risks and thorough pre-treatment evaluations contribute to more effective care, fostering improved relationships between providers and patients.
FAQs
1. What conditions is Cimzia approved to treat?
Cimzia is approved for the treatment of moderate-to-severe rheumatoid arthritis, plaque psoriasis, Crohn’s disease, psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis in adults.
2. How is Cimzia administered?
Cimzia treatment is administered through subcutaneous injections into the abdomen or thigh and is available as a lyophilized powder that requires reconstitution or as a prefilled syringe.
3. Are there any contraindications for using Cimzia?
Yes, Cimzia should not be given to patients with severe hypersensitivity to certolizumab pegol, those receiving live vaccines, or children due to unestablished safety and efficacy.
References
- Sullivan, H. W., Squire, C., Aikin, K. J., Tzeng, J., Ferriola-Bruckenstein, K., Brodsky, E., Trentacosti, A. M., & Johnson, M. (2022). Physicians’ use of and preferences for FDA-approved prescribing information. Research in social & administrative pharmacy: RSAP, 18(6), 3027–3037. https://doi.org/10.1016/j.sapharm.2021.07.028
- CIMZIA® HIGHLIGHTS OF PRESCRIBING INFORMATION. (n.d.-b). In CIMZIA. Retrieved January 28, 2025, from https://www.cimzia.com/themes/custom/cimzia/docs/CIMZIA_full_prescribing_information.pdf