Crohn’s disease affects millions of adults in the United States, with prevalence rates estimated between 2.4 and 3.1 million, according to the Centers for Disease Control and Prevention (CDC). As a chronic inflammatory condition, it can severely impact daily life, causing abdominal pain, persistent diarrhea, and extreme fatigue. Managing these symptoms effectively is essential to improving quality of life and long-term health outcomes.
For patients who haven’t found relief with conventional treatments, Cimzia, a biologic medication developed by UCB, offers a promising alternative. By targeting specific inflammatory proteins, Cimzia helps reduce symptoms and maintain disease control, giving patients a better chance at long-term remission. This treatment represents a significant advancement for those struggling with the unpredictable and often debilitating effects of Crohn’s disease.
In this article, we’ll take a closer look at how Cimzia works, its effectiveness in managing Crohn’s disease, potential side effects, and what patients can expect from this treatment.
Key Takeaways
- Cimzia is a biologic medication that targets and blocks Tumor Necrosis Factor (TNF) to reduce inflammation in adults with moderate to severe Crohn’s disease.
- Clinical trials have shown that 64% of patients experienced positive results within six weeks of starting Cimzia treatment.
- The typical dosing involves two 200 mg injections at the beginning and maintenance doses every four weeks for ongoing management.
- While Cimzia can significantly improve symptoms and quality of life, patients should be aware of potential side effects and consult their healthcare providers for optimal results.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Cimzia at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Cimzia’s Role in Treating Crohn’s Disease
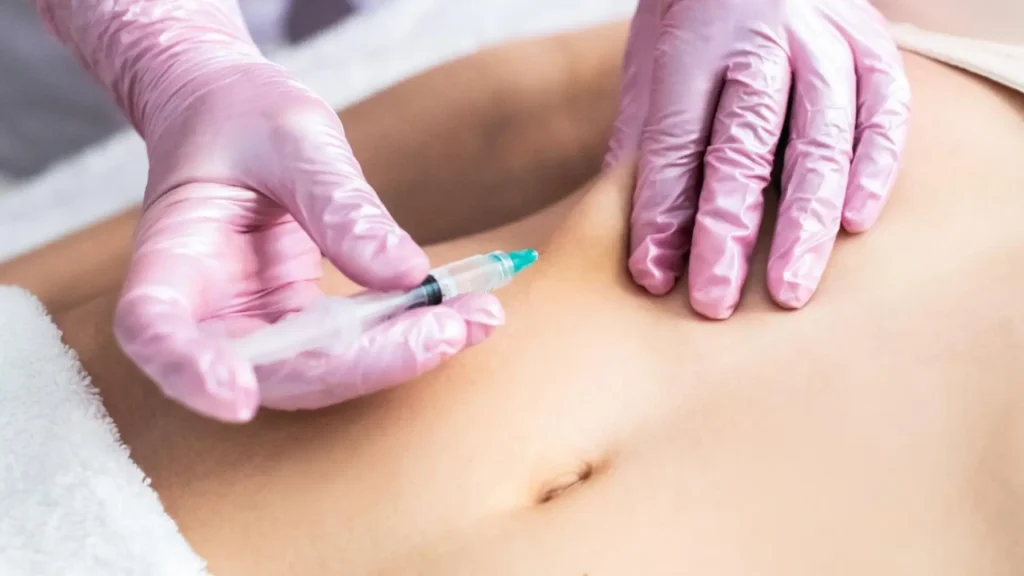
This minimally invasive biologic medication has been approved by the U.S. Food and Drug Administration (FDA) and other global regulatory agencies, earning the trust of both healthcare practitioners and patients worldwide. Cimzia’s manufacturer, UCB, remains committed to rigorous research and development, continuously working to create innovative therapies that enhance patients’ quality of life.
Cimzia (certolizumab pegol) is specifically designed to treat moderate to severe Crohn’s disease in adults. As a Tumor Necrosis Factor (TNF) blocker, it targets and neutralizes TNF, a key protein in the inflammatory response. By reducing inflammation in the digestive tract, Cimzia helps relieve symptoms and improve disease management.
Clinical trials have demonstrated Cimzia’s effectiveness in Crohn’s disease treatment. At six weeks, 64% of patients showed improvement, and by 26 weeks, 48% achieved remission. These results highlight Cimzia’s safety and efficacy, significantly improving disease activity and overall patient well-being.
Information on Dosing Schedules and Expected Results
The administration involves subcutaneous injections into the recommended Cimzia injection sites: abdomen or thighs. The maintenance dosing may depend on the severity of the condition and treatment plan, while the injection sessions are typically given every two to four weeks. The typical starter and maintenance dosing of Cimzia for Crohn’s disease include:
- Starter Dosing: Administer 200 mg twice at weeks 0, 2, and 4. This phase is essential to achieve the optimal medication levels in the patient’s system.
- Maintenance Dosing: Afterward, it should be administered under the skin as two separate 200 mg injections, taken every four weeks (or 28 days).
Patients may be trained to self-administer the injections at home or may receive them at a healthcare facility. The expected results from Cimzia treatment include significant improvements in disease activity and quality of life for patients with Crohn’s disease.
The positive results of clinical trials involving Cimzia for Crohn’s disease underscore the effectiveness of the injectable in managing this condition and enhancing patients’ overall well-being.
Potential Reactions
However, practitioners must discuss the common side effects with patients, equipping them with clear and comprehensive information about the procedure. This allows patients to make informed treatment decisions for getting Cimzia for Crohn’s disease. The reported typical reactions include:
- Injection site reactions (redness, swelling, bruising)
- Upper respiratory infections
- Arthritis pain
- Urinary tract infections
Patients must follow their healthcare provider’s instructions and promptly report any adverse effects. Adhering to the dosing regimen, post-treatment care, and monitoring can help patients achieve optimal treatment outcomes.
Symptom Relief with Cimzia
The clinical trial also showed how the medication enhanced patients’ quality of life. Among individuals who responded at six weeks, 6 out of 10 Cimzia patients reported improved quality of life at six months in the following areas:
- Bowel symptoms
- Feelings of fatigue
- Emotional distress
- Engagement in social activities
It’s worth noting that results may vary, as each person taking Cimzia is unique and responds differently to therapy. The Cimzia mechanism of action helps alleviate the common symptoms of Crohn’s disease, such as abdominal pain, diarrhea, and fatigue.
Patients must seek the guidance and expertise of medical professionals to ensure safe and effective treatment procedures. The Cimzia manufacturer has also proven the medication’s safety in treating conditions among pregnant individuals, making it appealing to a broader market globally.
Integrating Cimzia into Long-Term Treatment Plans

Integrating Cimzia into long-term treatment plans for Crohn’s disease requires a comprehensive approach to disease management. This biologic medication reduces inflammation and maintains clinical response in patients with moderate to severe Crohn’s disease. This consistent dosing schedule sustains the therapeutic effects of Cimzia and effectively manages Crohn’s disease symptoms.
To maximize Cimzia in disease management strategy, healthcare providers and patients must collaborate. The strategy may include:
- Regularly monitoring the patient’s response to treatment
- Adjusting the dosing regimen as needed
- Addressing any potential side effects
Additionally, patients should adopt lifestyle changes that support overall health, such as maintaining a balanced diet, managing emotional well-being, and staying physically active. Combining Cimzia with other therapeutic approaches and lifestyle modifications helps patients with Crohn’s disease achieve better disease control and improved long-term outcomes.
Conclusion
Cimzia represents a significant advancement in Crohn’s disease treatment, providing a targeted approach to managing symptoms and improving patients’ quality of life. By inhibiting TNF, Cimzia helps reduce inflammation, alleviate discomfort, and promote long-term disease control. Clinical trials further highlight its effectiveness, with many patients experiencing sustained symptom relief and remission.
However, successful treatment requires close collaboration with healthcare providers. Patients should have a clear understanding of the dosage regimen, potential side effects, and personalized treatment plans to achieve the best possible results while ensuring safety and long-term disease management.
FAQs
1. What is Cimzia, and how does it work for Crohn’s disease?
Cimzia (certolizumab pegol) is a biologic medication designed to treat moderate to severe Crohn’s disease in adults. It works as a Tumor Necrosis Factor (TNF) blocker, targeting and neutralizing TNF, a protein involved in the inflammatory process. This helps reduce inflammation in the digestive tract and alleviate symptoms associated with Crohn’s disease.
2. What is the typical dosing schedule for Cimzia?
The typical dosing for Cimzia starts with 200 mg administered twice at weeks 0, 2, and 4. After this starter phase, maintenance dosing usually involves two 200 mg injections every four weeks. Patients may self-administer the injections or have them administered at a healthcare facility.
3. What are the common side effects of Cimzia?
Common side effects of Cimzia include injection site reactions (such as redness and swelling), upper respiratory infections, arthritis pain, and urinary tract infections. Patients should discuss potential side effects with their healthcare provider and report any adverse reactions promptly.
References
- U.S. Centers For Disease Control and Prevention. (2024, June 21). IBD Facts and Stats. Inflammatory Bowel Disease (IBD). https://www.cdc.gov/inflammatory-bowel-disease/php/facts-stats/index.html
- See CIMZIA In Action for Crohn’s Disease. (n.d.). CIMZIA. Retrieved January 30, 2025, from https://www.cimzia.com/crohns-disease/how-cimzia-works