McKinsey research predicts that the global market for aesthetic injectables could grow by 12–14% annually over the next five years, driven by rising demand for minimally invasive treatments. This trend underscores the growing interest in non-surgical solutions for skin rejuvenation and beauty enhancement.
CG Bio Co., Ltd. has earned recognition as a leading innovator in biomaterial technology, particularly with its VOM fillers. Renowned for safety and efficiency, these hyaluronic acid injectables have become trusted solutions for individuals seeking effective and natural-looking results.
This article will introduce CG Bio Co., Ltd., the VOM Filler manufacturer, its history, innovative dermal fillers, commitment to quality, and unique hyaluronic acid formulations.
Key Takeaways
- CG Bio Co. Ltd. specializes in developing and manufacturing advanced medical devices and bioproducts, strongly focusing on wound management and orthopedic applications.
- Their research and development efforts focus on biomaterials, tissue engineering, and regenerative medicine. This focus ensures they deliver cutting-edge solutions for medical needs.
- This foray into medical aesthetics enabled them to pioneer advanced hyaluronic acid-based filler formulations, employing patented R-square technology to develop a multi-layered phasic filler.
- The company is still awaiting FDA and CE approval for its VOM fillers, which would enable it to access a broader aesthetic market.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Order VOM Filler online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
A Brief History of CG Bio Co., Ltd

CG Bio Co., Ltd., founded in 2006 in South Korea, has established itself as a leader in the biotechnology and medical device industry. Equipped with state-of-the-art facilities, including class 100 clean rooms and advanced purification systems for injectable products, the company delivers high-quality solutions in wound care, orthopedics, and aesthetics.
Over the years, CG Bio has achieved significant milestones. These milestones include obtaining CE certification for its hyaluronic acid-based dermal fillers, VOM and GISELLELIGNE, in 2021. The company has also expanded its global presence, with subsidiaries in the United States and Indonesia. This expansion reflects its commitment to innovation and international growth.
With a strong emphasis on safety and quality, CG Bio continues to develop cutting-edge products that meet the needs of both medical professionals and patients.
Commitment to Quality
CG Bio Co., Ltd. makes substantial investments in research and development to create innovative bio-products. Their R&D efforts are concentrated on biomaterials, tissue engineering, and regenerative medicine, ensuring they provide cutting-edge solutions for medical needs.
This company’s laboratory uniquely possesses the tissue scaffold, growth factor, and cell solution biotechnology essential for tissue regeneration. Thanks to its certifications and approvals, medical professionals can trust that it implements stringent quality control measures. Rigorous testing and validation processes uphold high product safety and efficacy standards.
Furthermore, these also showcase the safety and efficacy of the products in their indications. CG Bio Co., Ltd. adheres to international safety and efficacy standards, securing certifications from regulatory bodies like the CE. This ensures global compliance and enhances marketability.
Contributions to the Medical Aesthetics Field
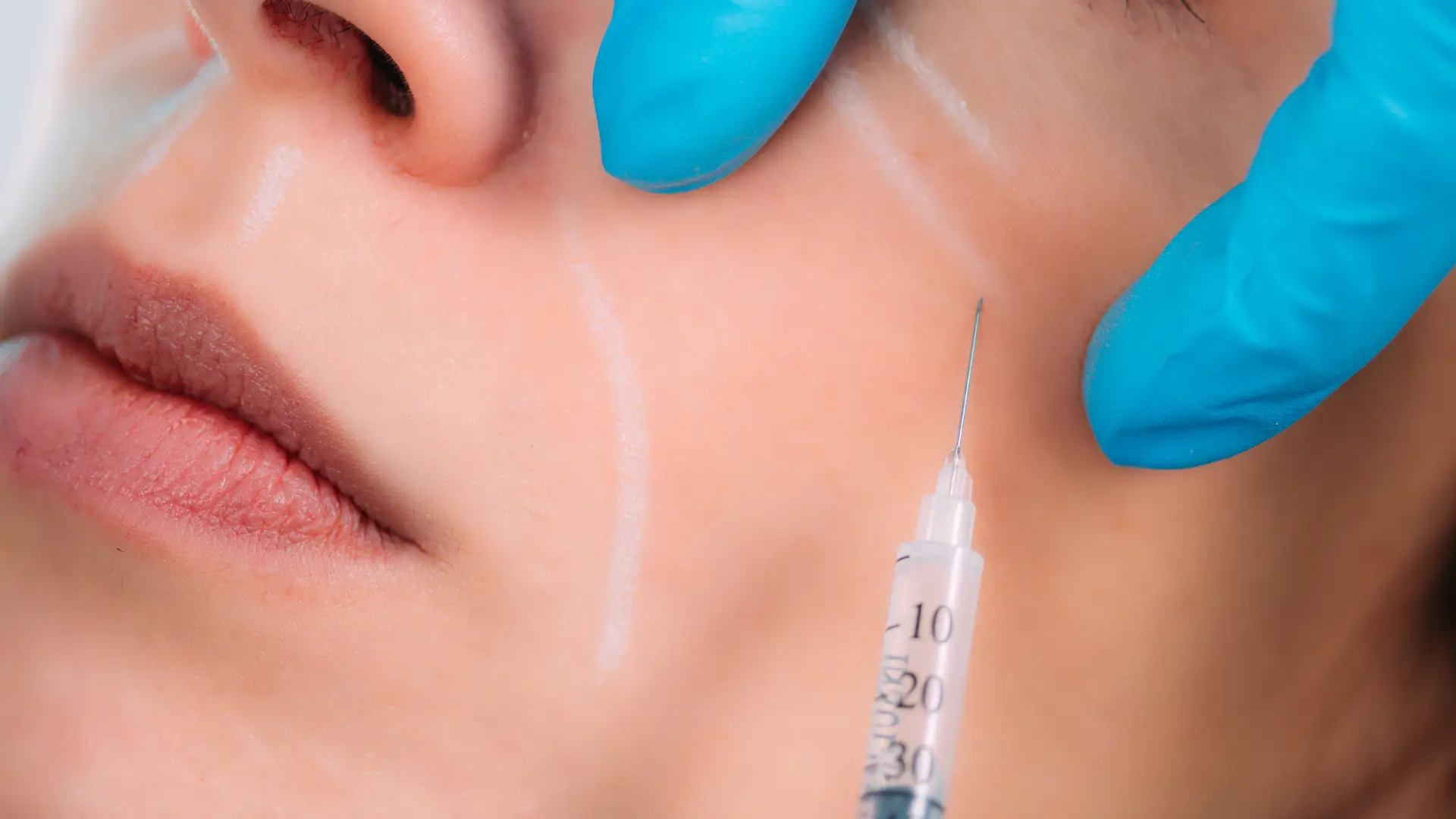
While initially focused on healthcare, CG Bio Co., Ltd. has made significant strides in the medical aesthetics field, pioneering advanced hyaluronic acid-based filler formulations. The company developed multi-layered phasic fillers through their patented R-square technology, ensuring even dispersion of crosslinked gel for superior safety and precision.
The VOM filler collection, which includes VOM V, VOM O, and VOM M, has become a trusted solution for facial rejuvenation and lip enhancement. These fillers, including VOM filler for lips, offer tailored results for various aesthetic concerns, from smoothing fine lines to enhancing lip volume. Their unique formulation elevates the safety and efficacy of treatments, earning recognition among aesthetic professionals.
Conclusion
CG Bio Co., Ltd. has emerged as a trailblazer in developing and producing innovative medical devices and aesthetic solutions, with VOM fillers leading the way. By leveraging advanced biomaterial technologies, the company has set new benchmarks in safety, efficacy, and patient satisfaction, addressing the growing demand for non-surgical treatments in the medical aesthetics field.
With its unwavering commitment to quality and research, CG Bio is poised for global expansion. The anticipated FDA and CE approvals for VOM fillers will further enhance their accessibility, solidifying the company’s role in shaping industry standards and delivering transformative outcomes for patients worldwide.
FAQs
1. What is CG Bio Co., Ltd. known for?
CG Bio Co., Ltd. specializes in developing and manufacturing advanced medical devices and bioproducts, mainly focusing on wound management and orthopedic applications. It is also recognized for its innovative aesthetic products, primarily VOM fillers based on hyaluronic acid.
2. What are VOM fillers?
VOM fillers are advanced hyaluronic acid-based injectables CG Bio Co., Ltd developed. They utilize patented R-square technology to create multi-layered phasic fillers, offering practical solutions for skin rejuvenation, particularly for the lips and other facial areas.
3. What regulatory approvals does CG Bio Co., Ltd. have for its products?
CG Bio Co., Ltd. has received CE certification for its medical HA fillers, VOM and GISELLELIGNE. However, they are still awaiting FDA approval for their VOM fillers to access a broader aesthetic market.
References
- Leclerc, O., Peters, N., Scaglione, A., & Waring, J. (2021, December 20). From extreme to mainstream: The future of aesthetics injectables | McKinsey. Www.mckinsey.com. https://www.mckinsey.com/industries/life-sciences/our-insights/from-extreme-to-mainstream-the-future-of-aesthetics-injectables
- Introduce – CGBIO_EN. (n.d.). Www.cgbio.co.kr; CG Bio Co. Ltd. Retrieved November 21, 2024, from https://www.cgbio.co.kr/en/company/introduce