Exercise and weight management are key strategies for managing osteoarthritis (OA), but recent research published in Arthritis & Rheumatology shows that hyaluronic acid (HA) injections can also significantly relieve pain and improve joint function. This has led to a rise in viscosupplementation as a temporary but effective solution for knee OA.
Among available viscosupplements, Supartz stands out as a promising treatment that leverages HA’s natural role as a lubricant and shock absorber in healthy joints. Its lasting effects aim to reduce osteoarthritis symptoms and enhance quality of life.
This article provides comprehensive prescribing information on Supartz, covering dosage guidelines, administration methods, contraindications, and essential safety considerations.
Key Takeaways
- The US Food and Drug Administration has approved Supartz for knee osteoarthritis patients who have not received relief from first-line treatments, such as oral analgesics or physical therapy.
- The recommended Supartz dosage is 2.5 mL injection per knee, requiring three to five weekly sessions for a complete treatment cycle.
- Patients should avoid self-administering Supartz and instead seek administration from a trained healthcare provider.
- After treatment, patients should anticipate common Supartz side effects, often experiencing mild to moderate reactions at the injection site.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Supartz wholesale at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
What is Supartz?
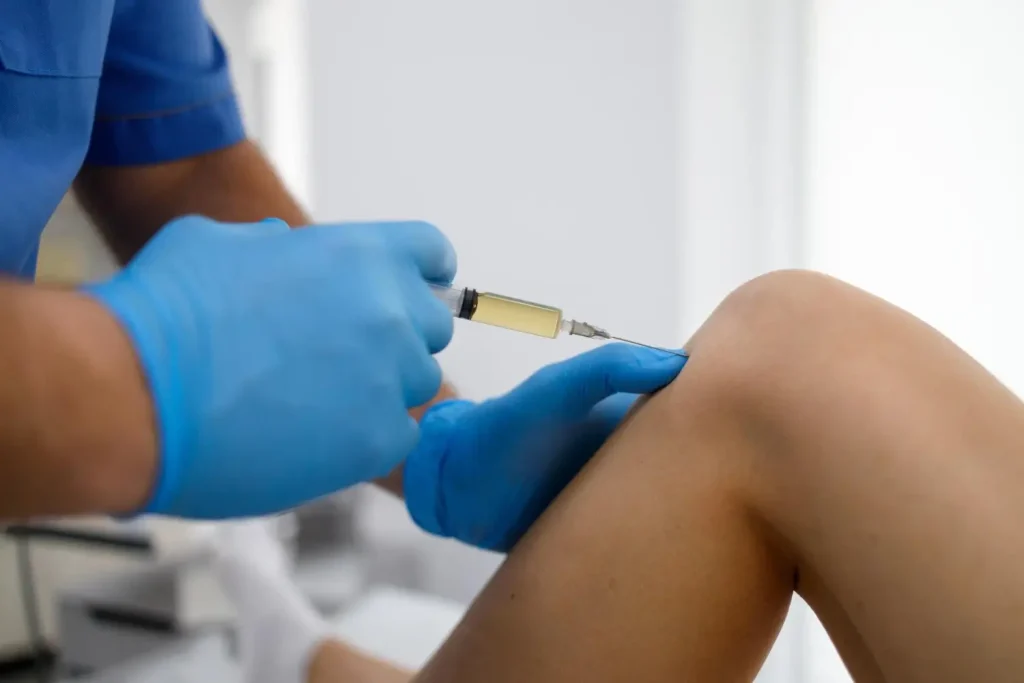
Supartz is a viscosupplement developed by Japan’s Seikagaku Corporation to relieve knee osteoarthritis (OA) symptoms. This injectable contains purified, high-molecular-weight hyaluronic acid (HA), mimicking the lost HA in the joint’s synovial fluid. This fluid lubricates and cushions joints for pain-free and better movement.
While other medical professionals may utilize Supartz for different joints, the U.S. Food and Drug Administration has approved it specifically for knee OA in patients who haven’t found relief with first-line therapies, such as pain relievers or physical therapy.
Designed to restore the viscoelastic properties of synovial fluid in the knee, Supartz helps reduce pain and improve joint mobility. Whether comparing Supartz vs Synvisc, patients can maximize the benefits of these viscosupplements in symptomatic relief and long-lasting results when injected directly into the affected knee joint by a qualified medical provider.
Dosage Recommendations
For knee osteoarthritis, the recommended Supartz dosage is 2.5 mL injected directly into each affected knee. Medical professionals should use a separate syringe for each knee to reduce the risk of complications.
The typical treatment schedule for Supartz involves three to five weekly injections to achieve lasting relief, although some patients start noticing improvements after just three sessions. Depending on individual factors like the severity of symptoms and overall joint health, the effects of Supartz can last for up to six months or longer.
A thorough consultation with a healthcare provider is critical, especially for older adults or patients with other medical conditions. In these cases, doctors may adjust the dosage to ensure the treatment is both safe and effective, tailoring it to each patient’s unique needs.
Administration Routes
To ensure safety and effectiveness, Supartz must be administered according to FDA guidelines and the official prescribing information. A trained medical professional should inject Supartz directly into the knee joint (intra-articularly), targeting the synovial space to maximize relief.
The injection is done under sterile conditions, carefully positioning Supartz within the joint for the best possible results. Some providers may also use Supartz for off-label areas, like the hip or shoulder, which requires extra precision and assessment to maintain safety.
Supartz should also be stored at room temperature, protected from light and moisture, and kept unfrozen to preserve quality. Importantly, patients should not self-administer Supartz—this treatment requires the expertise of a healthcare provider for safe and effective application.
Contraindications and Precautions

Before starting Supartz injections, healthcare providers should review key contraindications and precautions with patients to ensure safety and avoid complications.
- Avoid administering to patients with known allergies to sodium hyaluronate preparations.
- Refrain from injecting this product into the knees of patients with infections or skin diseases at the injection site.
In addition to these contraindications, patients should be aware that certain groups should exercise caution with Supartz.
- Pregnant Women: rephrase and improve: The safety and effectiveness of Supartz have not been established in pregnant women. The effectiveness of Supartz repeat treatment cycles has not been established.
- Nursing Mothers: It remains unclear whether Supartz is excreted in human milk, although it has been detected in rat milk. The safety and efficacy of Supartz in lactating women have not been established.
- Pediatrics: The safety and efficacy of Supartz in children have not been established.
Potential Drug Interactions and Adverse Effects
While there’s no current data on drug interactions with Supartz, providers should avoid using disinfectants containing quaternary ammonium salts for skin prep, as these can react with sodium hyaluronate and cause it to precipitate.
After injection, patients may experience mild to moderate side effects at the injection site, which usually resolve over time. Common temporary reactions include:
- Pain
- Swelling
- Effusion
- Redness
- Warmth
Less common adverse effects may include itching, facial swelling, rash, nausea, or fever. Patients experiencing persistent or severe symptoms should contact their healthcare provider immediately for further evaluation and management.
Safety Considerations

After completing a Supartz treatment cycle, patients should feel comfortable discussing concerns or potential risks with their healthcare provider. Practitioners encourage patients to monitor for any unusual reactions and report these promptly to ensure safe, effective therapy.
Regular follow-up appointments allow healthcare providers to assess how well the treatment is working and address any concerns that may arise. If adverse reactions occur, it’s important to consult a healthcare provider right away—they may adjust the treatment plan, discontinue use, or provide symptom relief to prevent complications.
Supartz’s effects can last up to six months, and many patients choose maintenance treatments to continue their results. Studies show that long-term use of Supartz is safe and effective, with repeat treatments often providing ongoing pain relief and improved joint mobility.
Conclusion
Supartz offers a non-surgical treatment option for individuals suffering from knee osteoarthritis who have not found relief from traditional therapies. Its high-molecular-weight hyaluronic acid formulation helps restore the joint’s natural cushioning, improving pain management and mobility. Patients can make informed decisions about their treatment options by understanding the proper dosage, protocols, and contraindications.
As with any medical intervention, thorough consultations with healthcare providers are essential to ensure suitability and safety. By adhering to the prescribing information and monitoring for potential risks, patients and providers can enhance the effectiveness of Supartz treatments and improve outcomes.
FAQs
1. What is Supartz, and how does it work?
Supartz is a high-molecular-weight hyaluronic acid injectable treatment designed to alleviate pain and improve function in patients with knee osteoarthritis. It mimics the natural hyaluronic acid in joint fluid, restoring lubrication and cushioning in the knee joint.
2. How is Supartz administered, and what is the recommended dosage?
A qualified healthcare provider administers Supartz injections into the affected knee joint. The recommended dosage is 2.5 mL per knee, and the typical treatment schedule is three to five weekly sessions.
3. Are there any contraindications or precautions for using Supartz?
Yes, Supartz should not be used in patients with known allergies to sodium hyaluronate or those with infections or skin diseases at the injection site. Special precautions are also advised for pregnant women, nursing mothers, and children, as the safety and efficacy in these populations have not been established.
References
- American College of Rheumatology. (n.d.). Osteoarthritis. Rheumatology.org. Retrieved October 30, 2024, from https://rheumatology.org/patients/osteoarthritis
- SUPARTZ FX (sodium hyaluronate). (n.d.). Bioventus LLC. Retrieved October 30, 2024, from https://www.oakneepainrelief.com/wp-content/uploads/2019/09/Supartz_FX_IFU.pdf