Medication quality and authenticity are vital to patient safety. According to the World Health Organization, an estimated 10.5% of medical products in low- and middle-income countries are substandard or falsified — a serious reminder of why sourcing treatments from verified pharmaceutical manufacturers matters.
At the forefront of trustworthy drug production is Novo Nordisk, the global company behind Ozempic (semaglutide). With over 100 years of innovation in diabetes care, Novo Nordisk has earned a reputation for rigorous quality standards, operating under Good Manufacturing Practice (GMP) guidelines and maintaining approvals from leading regulatory agencies worldwide. Though product indications and availability may vary by region, the company’s commitment to safety, consistency, and transparency remains constant.
In this article, we’ll take a closer look at Novo Nordisk’s role as the manufacturer of Ozempic, explore its global regulatory and quality frameworks, and discuss why these safeguards matter for clinicians and patients seeking reliable diabetes and weight management care.
Key Takeaways
- Novo Nordisk, the manufacturer of Ozempic, has been a global leader in diabetes care for over a century. It began with its groundbreaking work in insulin production.
- The company developed semaglutide, the active ingredient in Ozempic and Wegovy. It mimics the body’s natural GLP-1 hormone to regulate blood sugar and appetite.
- Novo Nordisk continues to innovate in drug delivery, creating user-friendly devices that improve accuracy, comfort, and adherence.
- Through ongoing research, post-marketing surveillance, and clinician education, Novo Nordisk ensures the safety, quality, and effectiveness of its products.
- Patients and providers benefit from the company’s commitment to ethical manufacturing, global regulatory standards, and continued advancements in metabolic health care.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Ozempic, contact Medica Depot’s sales representatives and they will guide you on how to do so. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
A Brief History of Novo Nordisk as a Leader in Diabetes Care
The story of Novo Nordisk dates back to the early 1920s, when August Krogh, inspired by his wife Marie Krogh’s advocacy for diabetes care, collaborated with Hans Christian Hagedorn to bring insulin to Denmark. What began as a small scientific mission quickly evolved into a pioneering force in global diabetes treatment. Over the decades, the company has expanded its focus from insulin production to tackling a range of chronic and metabolic diseases.
In 1923, the Nordisk Insulinlaboratorium successfully commercialized insulin production using advanced extraction and purification methods. This milestone transformed diabetes care and improved countless lives. By 1985, Novo Nordisk introduced the first NovoPen®, a major innovation in insulin delivery. This significantly helped patients self-administer medication more conveniently and accurately.

Today, the Danish pharmaceutical company continues to expand its expertise beyond diabetes, advancing treatments in metabolic and cardiovascular health, and in emerging areas such as NASH/MASH. Supported by extensive scientific research, Novo Nordisk drives major clinical programs exploring how semaglutide affects blood sugar, body weight, and cardiovascular outcomes.
For instance, the STEP clinical trials demonstrated that once-weekly semaglutide 2.4 mg, combined with lifestyle intervention, resulted in average weight reductions of nearly 15% at 68 weeks. These findings reinforce the long-term impact of semaglutide-based treatments—developed by Novo Nordisk, the manufacturer behind Ozempic and Wegovy—in managing both diabetes and obesity.
Development of Semaglutide and the Launch of Ozempic
Building on its long legacy in insulin innovation and peptide science, Novo Nordisk engineered semaglutide, the active ingredient in both Ozempic and Wegovy. This long-acting GLP-1 receptor agonist mimics the body’s natural incretin hormones, offering sustained activity and convenient once-weekly dosing.
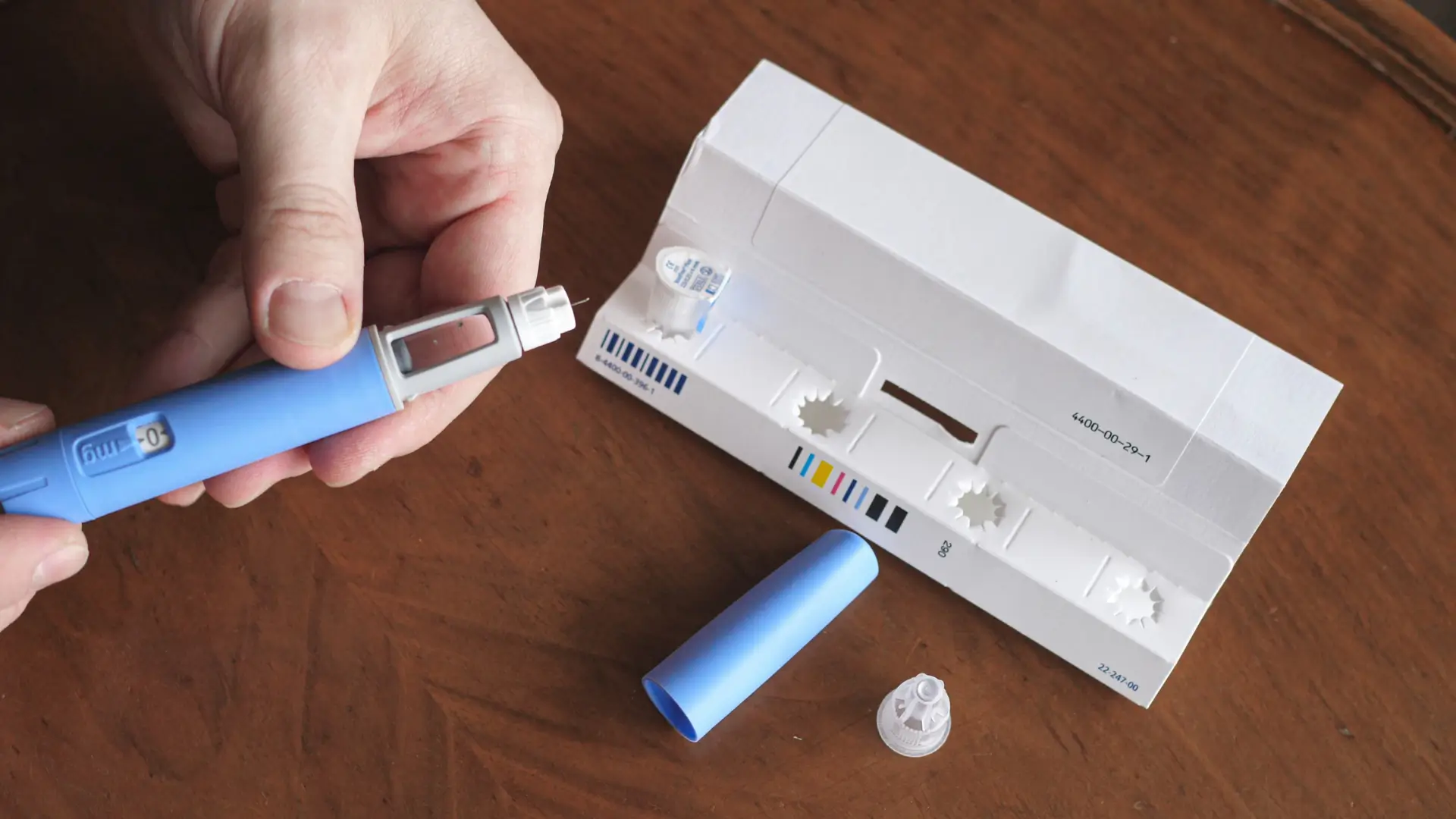
As Novo Nordisk introduced multiple semaglutide-based formulations, patients began asking, “Is Wegovy the same as Ozempic?” While both contain semaglutide, they differ in approved indications and dosing. Ozempic is indicated for type 2 diabetes and to reduce cardiovascular and kidney risks, while Wegovy is designed for chronic weight management in adults and adolescents with obesity or overweight conditions.
Ozempic remains one of the company’s flagship innovations — a treatment supported by FDA-approved prescribing information, clear patient-use instructions, and ongoing post-marketing surveillance. Novo Nordisk continues to emphasize safe administration, dose titration, and long-term monitoring to help patients achieve meaningful, sustained outcomes.
Innovation in Drug Delivery Devices by Novo Nordisk
Over the years, Novo Nordisk has reshaped how injectable medications are delivered, making them safer, more accurate, and patient-friendly. From pioneering the insulin pen in the 1980s to refining ergonomic design and precision dosing, the company has consistently prioritized patient comfort and usability.
These innovations carried forward into semaglutide-based injectables such as Ozempic, which come in prefilled, once-weekly pens designed for effortless use. These delivery systems minimize injection discomfort, reduce dosing errors, and help patients stay consistent with therapy. These are vital factors in long-term diabetes and metabolic management.
Commitment to Research, Safety, and Education With Ozempic

As the global manufacturer of Ozempic and Wegovy, Novo Nordisk places patient safety and education at the center of its mission. The company maintains active post-marketing surveillance, contributes to global safety registries. It collaborates with health authorities to ensure transparency and safety across all stages of the drug’s lifecycle.
In addition, Novo Nordisk provides comprehensive educational resources for both healthcare professionals and patients. This includes dosing guidance, titration instructions, and best practices for side-effect monitoring. By partnering with medical societies and academic institutions, the company helps integrate semaglutide therapies responsibly into modern diabetes and weight-management care.
Conclusion
For more than a century, Novo Nordisk has been at the forefront of endocrine and metabolic innovation, shaping how chronic diseases are managed worldwide. As the trusted manufacturer of Ozempic, the company continues to uphold its legacy of quality, safety, and scientific excellence.
Through breakthroughs like semaglutide and advancements in drug delivery technology, Novo Nordisk redefined standards of diabetes and obesity care. Their commitment to factors like patient outcomes and ethical manufacturing guarantees therapies like Ozempic remain both promising and patient-centered.
FAQs
1. Who manufactures Ozempic?
Ozempic is manufactured by Novo Nordisk. This global leader in diabetes care has a long history of developing innovative treatments for chronic conditions. The company is dedicated to improving patient outcomes through rigorous research and development.
2. What is the active ingredient in Ozempic, and how does it work?
The active ingredient in Ozempic, semaglutide, acts as a GLP-1 receptor agonist. This component lowers blood sugar levels in individuals with type 2 diabetes. It promotes insulin secretion, reduces appetite, and slows gastric emptying, all while being administered as a convenient once-weekly injection.
3. How does Novo Nordisk ensure the safety and efficacy of its products?
Novo Nordisk adheres to strict regulatory standards and Good Manufacturing Practices. They do this by conducting extensive clinical trials before product approval. The company also engages in continuous monitoring through post-marketing studies to assess long-term safety and effectiveness of its medications.
References
World Health Organization. Substandard and falsified medical products. who.int. Published November 26, 2019. Accessed October 10, 2025. https://www.who.int/teams/regulation-prequalification/regulation-and-safety/substandard-and-falsified-medical-products
Novo Nordisk. Our heritage | Novo Nordisk | Driving change. www.novonordisk.com. Accessed October 10, 2025. https://www.novonordisk.com/about/our-heritage.html