Did you know that 344 million people with osteoarthritis suffer from moderate to severe symptoms, many of whom could benefit from rehabilitation? This highlights the vast number of individuals affected by this degenerative joint disease who may be seeking effective medical treatments.
Knee osteoarthritis is especially common among both active and aging individuals, often causing joint pain, inflammation, and reduced mobility. Viscosupplements like Hymovis offer a promising solution, but it’s important to be aware of potential side effects.
In this article, we’ll provide a detailed overview of Hymovis side effects, including common and rare reactions, and how to manage them effectively.
Key Takeaways
- The Hymovis injection acts as a lubricant and shock absorber for the joints, providing symptomatic relief for several months.
- The Hymovis FDA approval only emphasizes the injection’s safety and efficacy in addressing knee OA among patients who failed to respond adequately to conservative first-line therapies.
- While Hymovis injection reviews showcase the injectable’s efficacy and safety, patients expect the occurrence of common side effects after injections.
- Equipping patients with comprehensive information about these risks can help them decide their treatments.
- Patients must adhere to these instructions to ensure safety, smooth recovery, and minimized risks throughout their Hymovis treatment.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Hymovis wholesale at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
What is Hymovis?
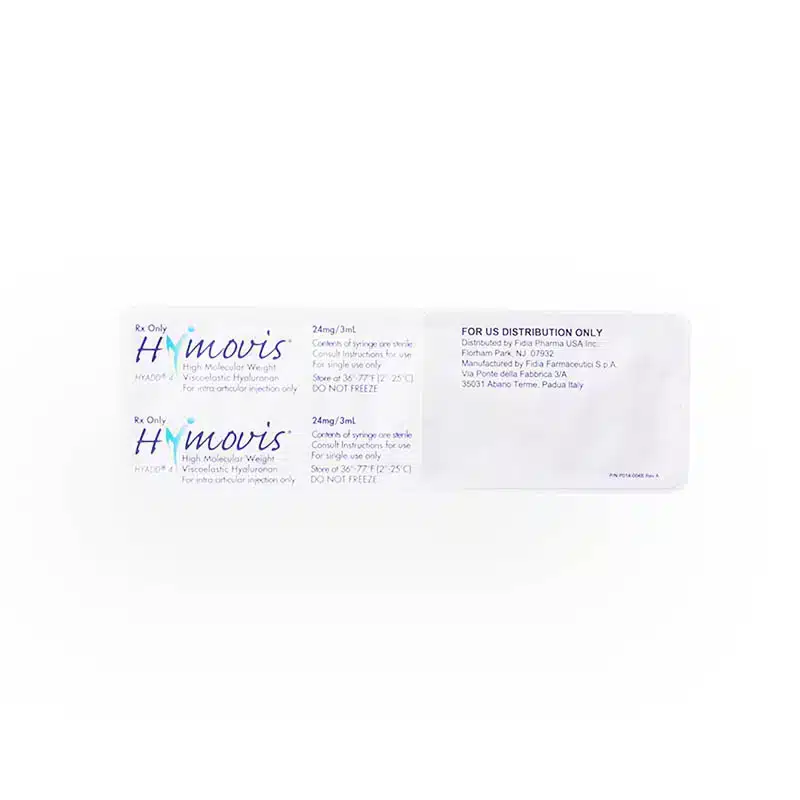
Hymovis is an FDA-approved injectable treatment specifically designed to treat knee osteoarthritis (OA) in individuals who have not found relief through first-line therapies, such as analgesics or physical therapy. This injectable capitalizes on its high-molecular-weight viscoelastic hyaluronan, similar to the joint’s natural synovial fluid. Hymovis utilizes a unique hydrogel formulation of HYADD®4, a molecule not chemically crosslinked.
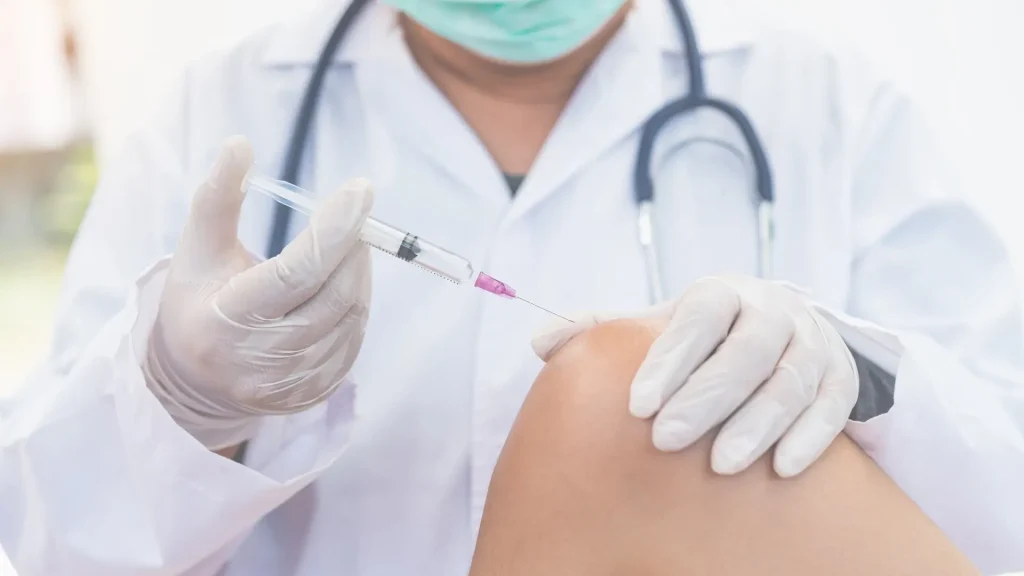
The typical treatment involves two injections administered by a healthcare provider, spaced one week apart. The injections are designed to reduce joint pain and improve function for several months.
While its FDA approval focuses on knee osteoarthritis, some healthcare providers may use Hymovis off-label to manage OA in other joints, such as the hips, hands, or shoulders, when conservative treatments have proven insufficient.
Common Side Effects of Hymovis
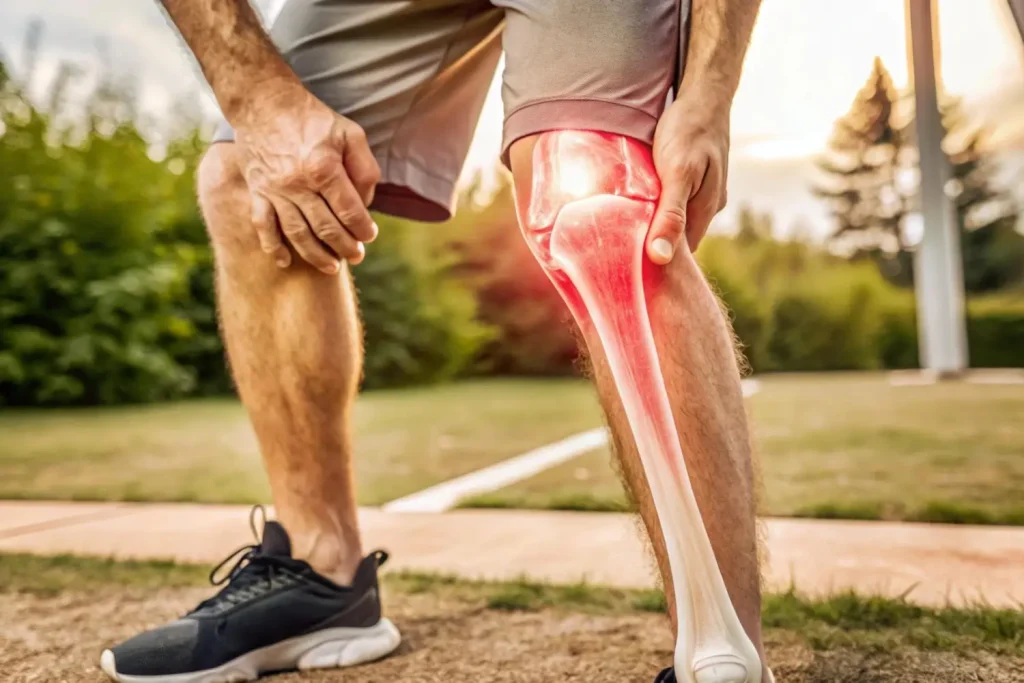
While Hymovis injection reviews showcase the injectable’s efficacy and safety, patients expect the occurrence of common side effects after injections. The typical symptoms are generally mild to moderate and may subside within a few days.
- Pain or Swelling at the Injection Site: Patients frequently experience pain, swelling, or redness at the injection site, which is the skin’s reaction to the trauma or injection process. This typically resolves after a few hours to a few days.
- Arthralgia: Hymovis reported that joint pain is patients’ most commonly reported adverse effect associated with intra-articular injection. Some may feel discomfort post-injection, and patients should understand that this is a common reaction to injections like Hymovis.
If these typical Hymovis side effects persist, patients should consult their healthcare providers for proper management for a safe and smooth recovery.
Uncommon and Rare Side Effects of Hymovis
Medical professionals should discuss the possibility of rare and severe Hymovis side effects. Equipping patients with comprehensive information about these risks can help them decide their treatments. Although infrequent, these symptoms may occur and necessitate immediate medical attention for prompt action and management.
- Allergic Reactions: When this side effect occurs, patients may notice symptoms such as hives, itchy skin, difficulty breathing, and swelling of the face, lips, tongue, or throat.
- Infection at the Injection Site: Patients must monitor the site for changes or symptoms like warmth, swelling, redness, and increased knee pain, as they may be signs of infection.
- Nerve Damage: While rare, providers and patients should understand that nerve damage may present as numbness, tingling, or weakness in the affected area or injection site.
Medical providers must explain the contraindications of Hymovis to patients to avoid severe complications, such as these rare symptoms. Avoiding administration to individuals with these conditions can minimize health and treatment risks.
- Patients with known hypersensitivity to hyaluronate preparations.
- Patients with known hypersensitivity to gram-positive bacterial proteins.
- Patients with infections or skin diseases in the area of the injection site or joint.
However, when rare Hymovis side effects still occur, patients should seek the help of their healthcare providers. These professionals can appropriately manage side effects to avoid further complications.
Managing Hymovis Side Effects
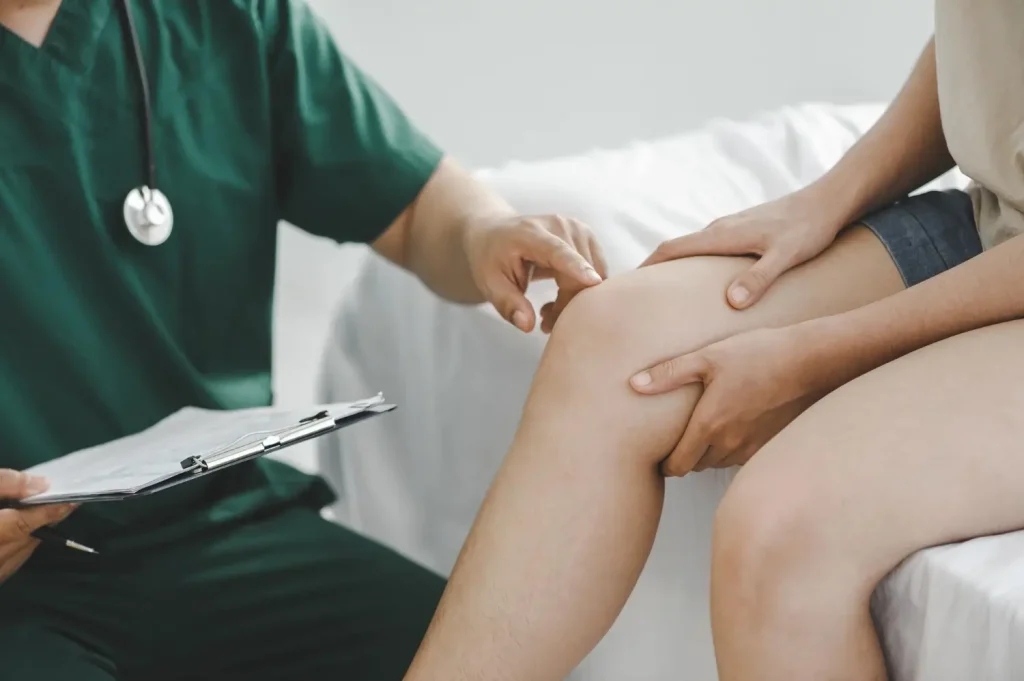
Furthermore, healthcare providers can offer personalized tips for managing common Hymovis side effects. Patients must adhere to these instructions to ensure safety, smooth recovery, and minimized risks throughout their Hymovis treatment.
- Apply Ice Packs
- Rest the Affected Joint
- Take Over-the-Counter Medications When Prescribed
- Maintain Proper Hydration and Lifestyle
- Avoid Strenuous Activities 48 Hours Post-Injection
These can help alleviate joint pain, stiffness, redness, or swelling at the injection site after the sessions. However, when patients experience severe symptoms, such as signs of allergic reactions, infection, or knee pain, they should immediately seek medical attention.
Individuals should discuss their concerns with their healthcare providers to relieve patients’ anxiety and answer their questions. Open communication between doctors and patients about the treatment and its risks can build trust and confidence in Hymovis.
Proper discussion of medical history, current medications, allergies, and knee osteoarthritis conditions can help providers create a tailored treatment plan for each patient. This personalized approach can ensure safe and effective Hymovis treatment.
Conclusion
Understanding the potential Hymovis side effects is essential for both patients and healthcare providers. By categorizing side effects into common, uncommon, and rare reactions, individuals can make informed decisions about their treatment. Close collaboration between patients and providers ensures side effects are monitored and managed, promoting a safe recovery.
Seeking medical attention when needed and following healthcare providers’ instructions can help maximize symptomatic relief. For those considering or undergoing Hymovis treatment, an informed approach to managing side effects is key to achieving the best possible outcomes for knee osteoarthritis patients.
FAQs
1. What is Hymovis used for?
Hymovis injections treat knee osteoarthritis in individuals who have not responded to other treatments, such as simple analgesics or physical therapy.
2. What are the common side effects of Hymovis injections?
Common side effects of Hymovis injections include pain or swelling at the injection site and arthralgia (joint pain).
3. What should patients do if they experience rare side effects of Hymovis?
If patients experience rare side effects of Hymovis, such as allergic reactions, infection at the injection site, or nerve damage, they should seek immediate medical attention for prompt action and management.
References
- World Health Organization. (2023, July 14). Osteoarthritis. Www.who.int; World Health Organization. https://www.who.int/news-room/fact-sheets/detail/osteoarthritis
- Hymovis Instructions For Use. (n.d.). In hcp.hymovis.com. Hymovis. Retrieved September 23, 2024, from https://hcp.hymovis.com/wp-content/uploads/sites/2/2023/02/Hymovis-Package-Insert-USA-68573-13-C-June-20211.pdf