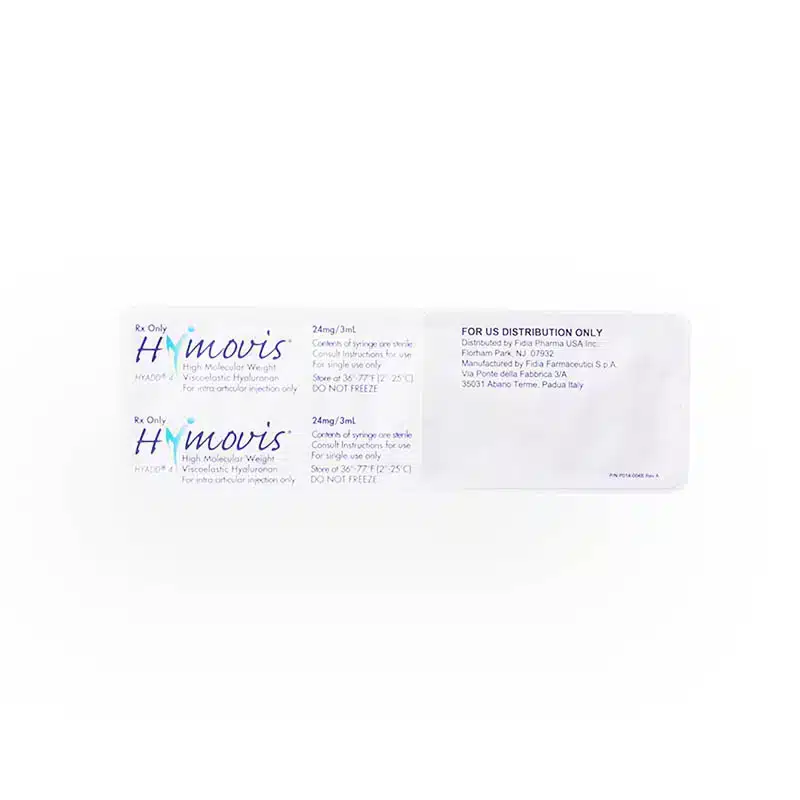
Clear and precise drug labeling is crucial for healthcare providers, particularly in FDA-approved prescribing information. According to a study by Sullivan et al. (2022), well-structured prescribing details significantly enhance the communication of vital drug information to physicians, ensuring safe and effective treatment.
Prescribing information is essential for healthcare providers to fully understand a product’s proper use and administration, ultimately improving patient outcomes. In the case of knee osteoarthritis, understanding the specifics of Hymovis can help physicians tailor treatments to provide optimal pain relief and improve the quality of life for their patients.
In this article, we will explore the Hymovis prescribing information, including dosage guidelines, administration techniques, and key contraindications.
Key Takeaways
- Hymovis injections restore the viscoelastic properties of the joint’s synovial fluid to provide lubrication and shock absorption.
- The typical dosage of Hymovis injection requires the full 3 mL for each knee.
- Medical professionals should administer Hymovis twice, given one week apart.
- Hymovis and other viscosupplements require intra-articular injection directly into the affected knee joint.
- Before proceeding with Hymovis treatment, medical providers should consider and evaluate the following factors to determine the most suitable treatment for knee osteoarthritis patients.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Hymovis online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
What is Hymovis?
Viscosupplementation has emerged as a popular non-surgical solution for knee osteoarthritis (OA) individuals. Among available options, Hymovis injections offer its unique hyaluronan derivative, HYADD®4. This comes in high-molecular-weight viscoelastic hydrogel to address symptoms caused by knee OA.
When comparing Hymovis vs Hyalgan or Hymovis vs Synvisc, it’s worth noting that these viscosupplements have similar mechanisms of action. When administered, these injectables restore the viscoelastic properties of the joint’s synovial fluid to provide lubrication and shock absorption.
With this mechanism of action, Hymovis injection reduces friction and cushions the joint during movement. This leads to improved mobility, minimized knee OA symptoms, and enhanced quality of life. Depending on dosage, administration, and individual factors, the Hymovis effects may last up to six months or longer.
Dosage Guidelines for Hymovis
A tailored treatment plan ensures patient suitability and safety for Hymovis injections. Medical providers can align Hymovis dosage based on each individual’s condition and medical history. They should discuss this factor with patients, allowing them to understand the dosage requirement for the severity of the condition and injection frequency for treatment protocol.
- Recommended Dosage: Hymovis is supplied in a 5 mL syringe containing a 3 mL solution. The typical dosage of Hymovis injection requires the full 3 mL for each knee.
- Administration Frequency: Medical professionals should administer Hymovis twice, given one week apart. This two-injection regimen aims to provide optimal symptomatic relief from knee osteoarthritis.
Administration Routes for Hymovis
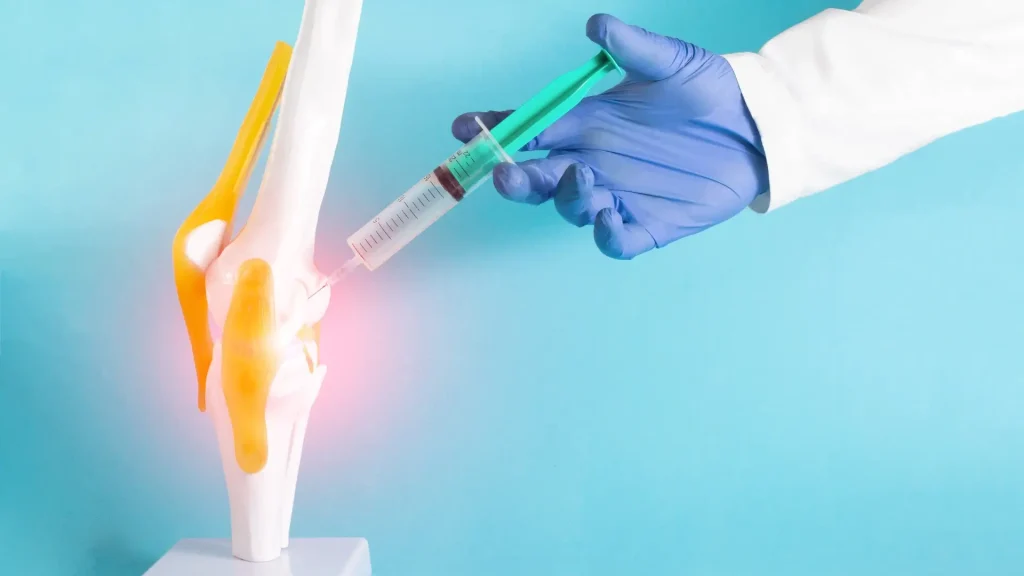
Thorough consultations with medical professionals involve equipping patients with comprehensive information about their treatment. Discussing important information empowers individuals to make informed treatment decisions and builds trust and confidence in Hymovis injections. Moreover, understanding the significance of proper administration techniques can help them set realistic expectations.
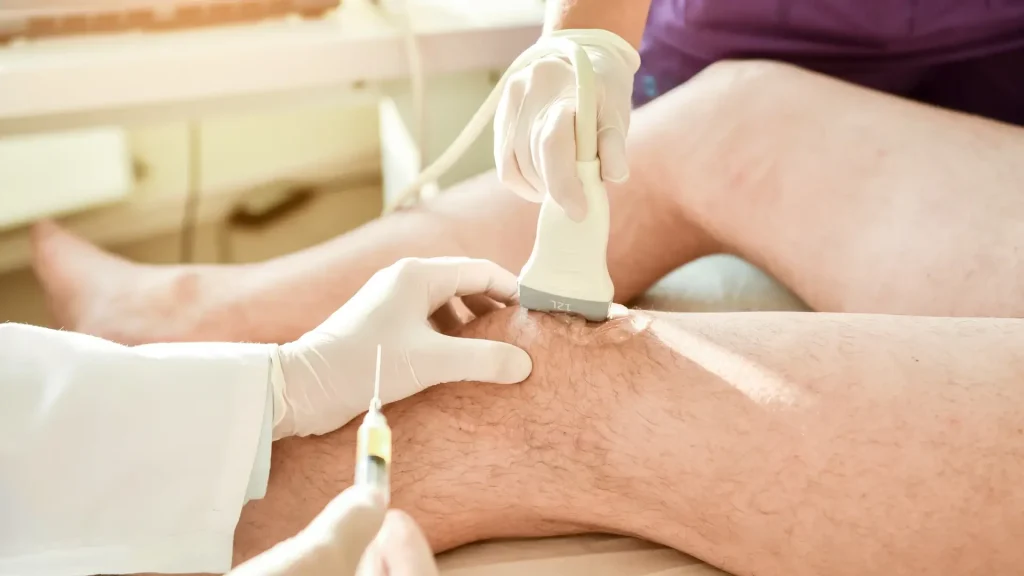
- Injection Technique: Hymovis and other viscosupplements require intra-articular injection directly into the affected knee joint. According to the Hymovis prescribing information, medical professionals should utilize a strict aseptic technique and use an 18-20 gauge needle.
- Site of Administration: Medical professionals should target the knee joint, with each knee requiring a separate syringe for individuals needing bilateral treatment.
The Hymovis FDA approval only indicates treatment for knee osteoarthritis. However, some medical practitioners may recommend off-label use of Hymovis for other joints, like the hip. Off-label uses may require a more meticulous evaluation to ensure patient safety throughout the treatment.
Contraindications and Considerations
A consultation with healthcare providers allows open communication with patients, understanding their condition and goals. Before proceeding with Hymovis treatment, medical providers should consider and evaluate the following factors to determine the most suitable treatment for knee osteoarthritis (OA) patients.
- Age
- Current Medications
- Medical History
- Previous Treatments
- Severity of Knee OA Condition
The adult population, aged 22 and above, can find viscosupplements beneficial. However, pediatric, pregnant, and breastfeeding individuals should avoid Hymovis due to its unestablished safety and efficacy for these populations. Moreover, medical professionals should also note the contraindications for the use of Hymovis.
- Do not administer to patients with known hypersensitivity to hyaluronate preparations.
- Avoid administering to patients with known hypersensitivity to gram-positive bacterial proteins.
- Do not administer to patients with infections or skin diseases in the area of the injection site or affected joint.
Patients may also seek maintenance treatments to sustain relief and enhanced quality of life. Understanding these factors can ensure patients achieve optimal symptomatic relief from their knee OA condition.
Conclusion
A thorough understanding of Hymovis prescribing information is essential for medical professionals aiming to optimize knee osteoarthritis (OA) treatment. Adhering to dosage guidelines, proper administration techniques, and recognizing contraindications enables informed decision-making and prioritizes patient safety.
With its unique hyaluronan derivative, HYADD®4, Hymovis offers a valuable non-surgical solution for managing knee OA symptoms. By following the recommended treatment protocol and injection procedures, healthcare providers can ensure safe, effective relief—ultimately improving mobility and enhancing the quality of life for OA patients.
FAQs
1. What is Hymovis?
Hymovis is a viscosupplement injection designed to relieve knee osteoarthritis by restoring the synovial fluid’s viscoelastic properties.
2. What are the dosage guidelines for Hymovis?
The typical dosage of Hymovis injection requires the full 3 mL for each knee, and medical professionals should administer Hymovis twice, given one week apart.
3. What factors should medical providers consider before proceeding with Hymovis treatment?
Before proceeding with Hymovis treatment, medical providers should evaluate the patient’s age, current medications, medical history, previous treatments, and the severity of the knee osteoarthritis condition.
References
- Sullivan, H. W., Squire, C., Aikin, K. J., Tzeng, J., Ferriola-Bruckenstein, K., Brodsky, E., Trentacosti, A. M., & Johnson, M. (2022). Physicians’ use of and preferences for FDA-approved prescribing information. Research in social & administrative pharmacy: RSAP, 18(6), 3027–3037. https://doi.org/10.1016/j.sapharm.2021.07.028
- Hymovis Instructions For Use. (n.d.). In hcp.hymovis.com. Hymovis. Retrieved September 26, 2024, from https://hcp.hymovis.com/wp-content/uploads/sites/2/2023/02/Hymovis-Package-Insert-USA-68573-13-C-June-20211.pdf