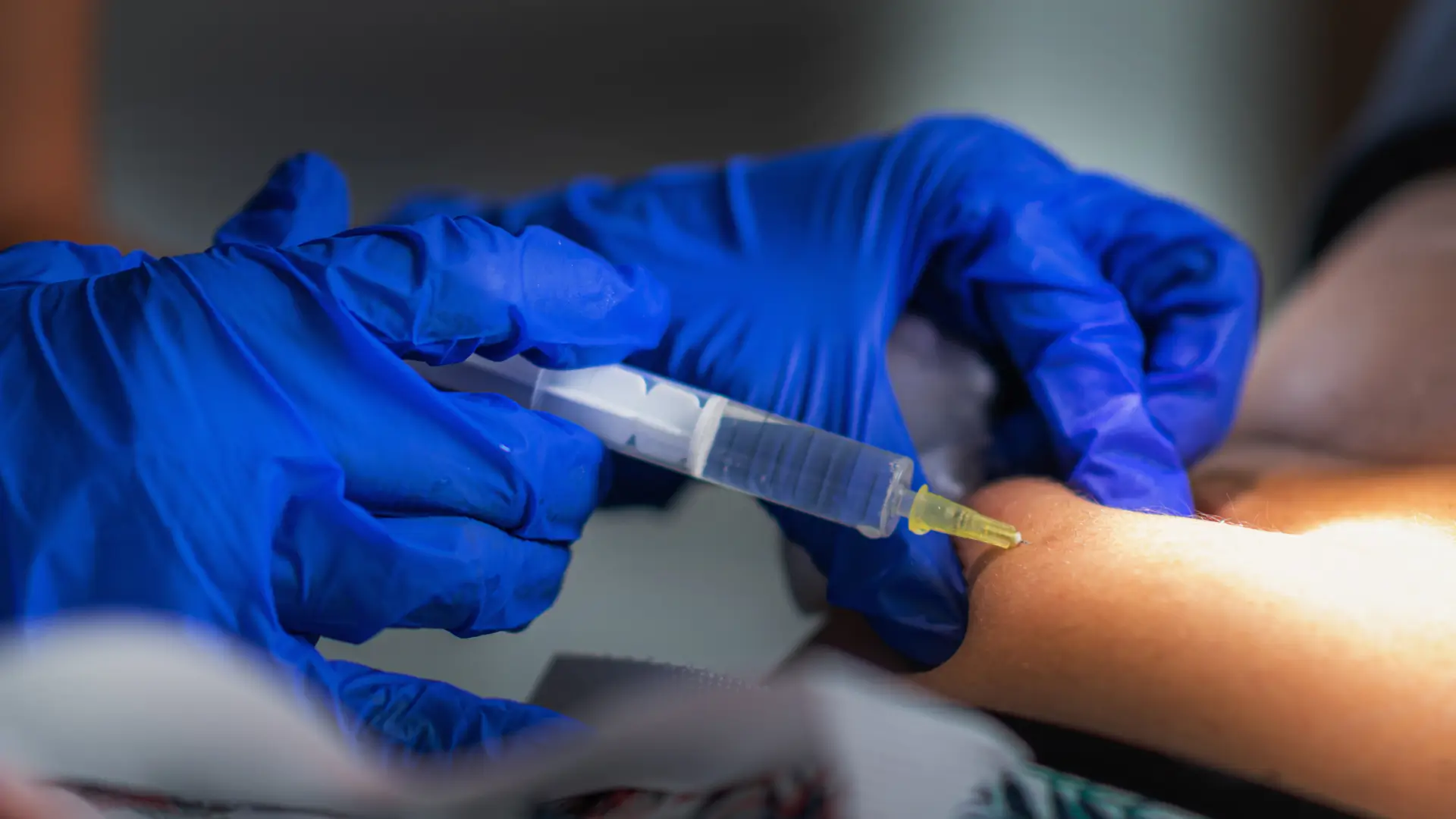
Minimally invasive injections have transformed osteoporosis treatment, providing a less painful and more convenient alternative to traditional therapies. These non-surgical treatments offer significant benefits, including reduced fracture risk and improved bone density, making them an essential option for many patients.
Prolia (denosumab), developed by Amgen, is a biannual injection designed to strengthen bones and lower the risk of fractures. Proper administration and dosing are crucial for achieving optimal results, ensuring patients receive the full benefits of this treatment.
In this article, we’ll explore how Prolia is administered, its benefits, potential side effects, and how it compares to other osteoporosis treatments.
Key Takeaways
- Prolia is administered as a 60 mg subcutaneous injection in areas like the upper arm, thigh, or abdomen, typically every six months.
- Proper injection techniques include cleansing the treatment area, allowing Prolia to reach room temperature, and ensuring the injection doesn’t hit a blood vessel.
- Healthcare professionals should rotate injection sites to minimize pain and discomfort, enhancing the patient experience during treatment.
- Patient education is vital for adherence, as it helps individuals understand the administration process, potential side effects, and the importance of follow-up appointments.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Our sales representatives can advise you on how to buy Prolia online today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Proper Techniques for Administering Prolia Injections
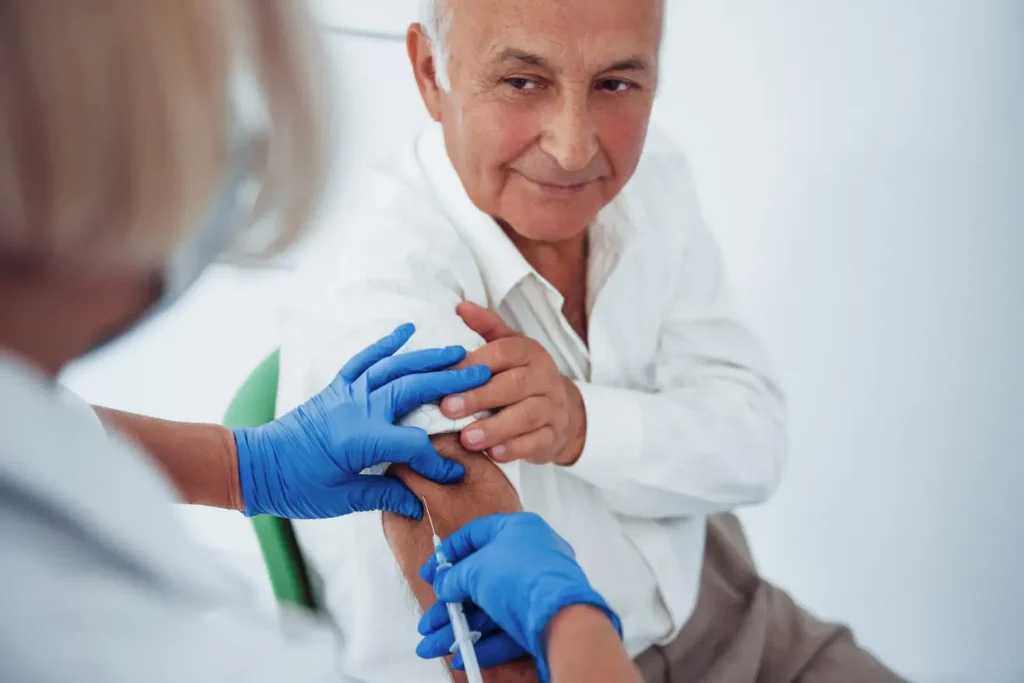
Seeking treatment from licensed medical professionals is essential for achieving optimal results with Prolia. This minimally invasive injection has FDA approval to treat several conditions, including:
- Postmenopausal osteoporosis in women at high risk for fractures.
- Osteoporosis in men at high risk for fractures.
- Glucocorticoid-induced osteoporosis in men and women at high risk for fractures.
- Bone mass loss in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer.
- Bone mass loss in women receiving adjuvant aromatase inhibitor therapy for breast cancer.
Prolia Dosing and Administration
The appropriate Prolia dosing is 60 mg, administered as a subcutaneous injection in one of the following sites:
- Upper arm
- Upper thigh
- Abdomen
Step-by-Step Administration Guide
Before administration, practitioners should remove Prolia from the refrigerator and allow it to reach room temperature (up to 25°C/77°F). This ensures proper preparation and reduces discomfort during injection. Medical providers must follow the official guidelines from Amgen and the FDA to ensure proper administration:
- Cleanse the injection site.
- Remove the gray needle cap.
- Administer the injection subcutaneously into the selected site, avoiding blood vessels.
- Ensure full delivery of the 60 mg dose.
- Activate the safety guard over the needle and dispose of it properly.
Preventing Common Injection-Related Issues
Following proper injection techniques can minimize complications and enhance patient comfort. Trained professionals should take additional precautions, including:
- Reducing pain: Rotate injection sites to prevent tissue irritation. Providers should consider patient preferences when selecting the next injection site.
- Managing bleeding and reactions: Apply gentle pressure to the site if bleeding occurs and provide post-treatment care instructions to prevent further complications.
Handling and Preparation of Prolia
Correct storage and handling of Prolia are essential for maintaining treatment efficacy and safety. Whether comparing Prolia vs Evenity, understanding the proper conditions for these medications is crucial.
According to Prolia’s prescribing information, practitioners should store this osteoporosis injectable in a refrigerator at 2°C to 8°C (36°F to 46°F) in its original carton to protect it from light.
Once removed from refrigeration, Prolia must not be exposed to temperatures above 25°C (77°F) and should be used within 14 days. If not administered within this period, it must be discarded.
Key Storage Guidelines
- Do not freeze the medication.
- Do not use Prolia past its expiration date as indicated on the label.
- Keep Prolia away from direct light and heat to prevent degradation.
- Avoid excessive shaking, as this can alter the formulation’s effectiveness.
Warming and Preparing the Injection
To ensure safety and efficacy, healthcare providers must follow these preparation steps:
- Allow Prolia to reach room temperature naturally before administration.
- Do not use external heat sources such as hot water or microwaves to warm the syringe.
- Inspect the syringe and medication for any signs of damage or discoloration before use.
Safety Precautions for Healthcare Providers
Once the prefilled syringe is ready for injection, the administering doctor should carefully remove the gray needle cap. After treatment, regular follow-up visits are essential for monitoring patient response, treatment progress, and potential risks.
Healthcare providers should
- Monitor calcium levels and prescribe calcium and vitamin D supplements as needed, especially for patients with chronic kidney disease.
- Be aware of serious risks, such as severe hypocalcemia, osteonecrosis of the jaw (ONJ), and atypical thigh bone fractures.
- Follow proper injection techniques to minimize discomfort and reduce common side effects like bruising or swelling.
- Educate patients on post-treatment care, good oral hygiene, and regular monitoring to ensure long-term safety and effectiveness.
Best Practices for Ensuring Patient Adherence

A licensed and trained healthcare professional plays a crucial role in administering Prolia safely and maximizing its benefits for osteoporosis treatment. Seeking their expertise ensures that patients receive proper guidance, safe treatment, and optimal outcomes.
Best Practices for Healthcare Providers
- Counseling Patients On Injection Schedules: Educate patients about Prolia’s dosing schedule and emphasize the importance of adhering to their injection timeline. Provide clear instructions, reminders for upcoming doses, and explain the risks of missed injections. Encouraging the use of calendar reminders or mobile alerts can help patients stay on track.
- Addressing Common Concerns And Misconceptions: Encourage open communication so patients can express concerns or misconceptions about Prolia. Healthcare providers should offer evidence-based information, reinforcing Prolia’s proven safety and effectiveness while addressing any doubts with credibility and clarity.
- Monitoring For Adverse Reactions Post-Injection: Scheduling follow-up appointments allows for early detection of side effects and ensures patient safety. Educate patients on what symptoms to watch for and when to seek medical attention. Additionally, provide guidance on reporting side effects, ensuring proactive management of any potential concerns.
Conclusion
Proper Prolia administration involves a simple yet precise process, requiring a 60 mg subcutaneous injection in the upper arm, thigh, or abdomen. Healthcare professionals must strictly follow storage and handling guidelines to maintain the injection’s effectiveness and safety.
Educating patients on administration, side effects, and aftercare promotes adherence and confidence in their treatment. By following best practices, healthcare providers help maximize Prolia’s benefits and overall patient outcomes.
FAQs
1. How is Prolia administered?
Prolia is administered as a 60 mg subcutaneous injection, typically in the upper arm, upper thigh, or abdomen. A licensed healthcare professional must give this injection to ensure safety and efficacy.
2. What should be done to prepare Prolia for injection?
Before administering Prolia, it should be removed from the refrigerator and allowed to reach room temperature (up to 25°C/77°F) for optimal preparation. It’s essential to cleanse the injection site and check that the syringe is free from damage.
3. What precautions should be taken during the injection process?
To prevent complications, healthcare providers should avoid injecting Prolia into blood vessels, manage any bleeding with gentle pressure, and rotate injection sites to minimize discomfort. Proper disposal of the needle and syringe is also crucial for safety.
References
- Luo, Y., Yang, D.-M., Yang, H.-M., Wu, D., & Xie, F.-Y. (2023). Innovative minimally invasive implants for osteoporosis vertebral compression fractures. Frontiers in Medicine, 10. https://doi.org/10.3389/fmed.2023.1161174
- HIGHLIGHTS OF PRESCRIBING INFORMATION Prolia ® (denosumab). (n.d.). Retrieved February 21, 2025, from https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Prolia/prolia_pi.pdf