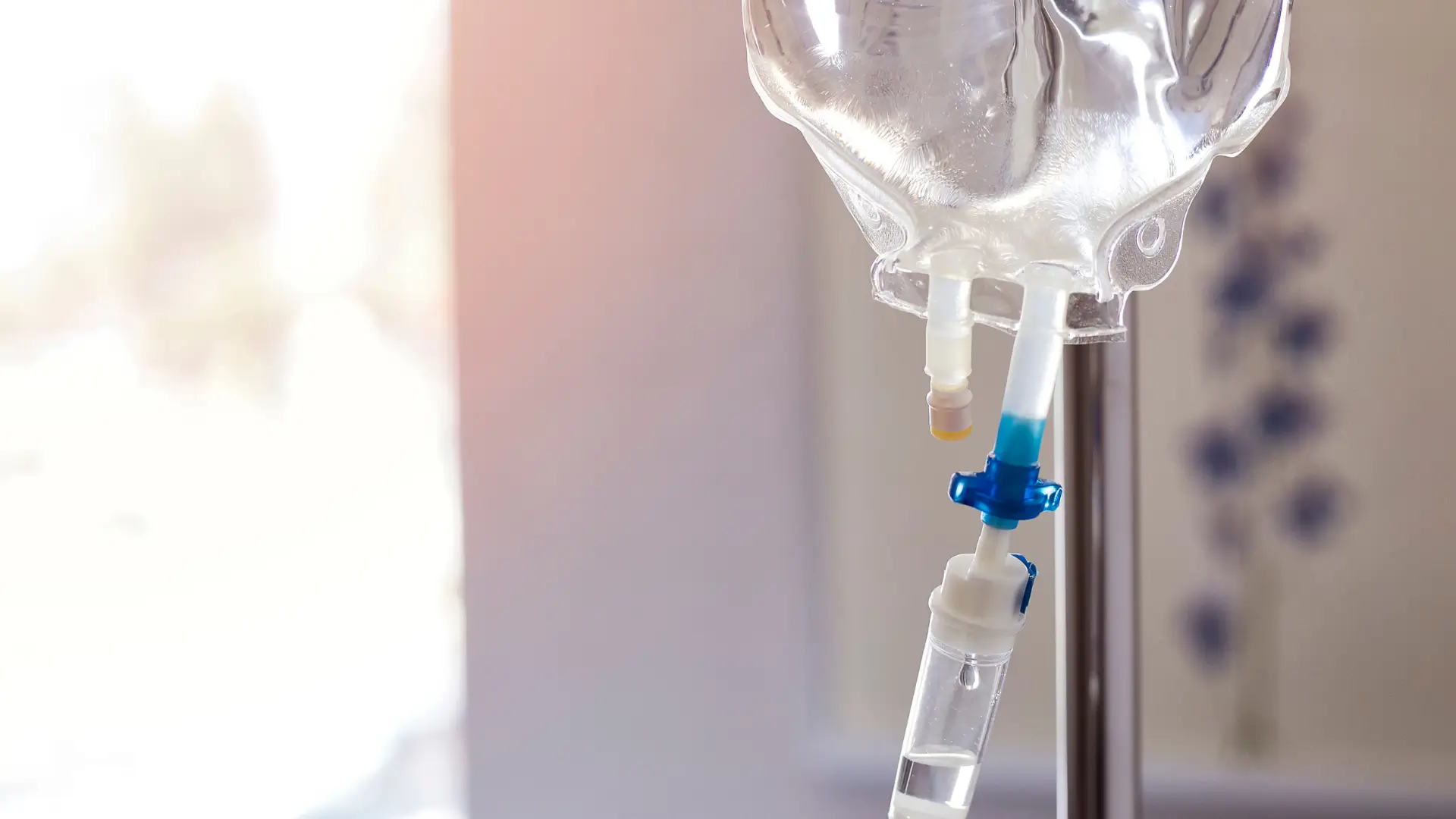
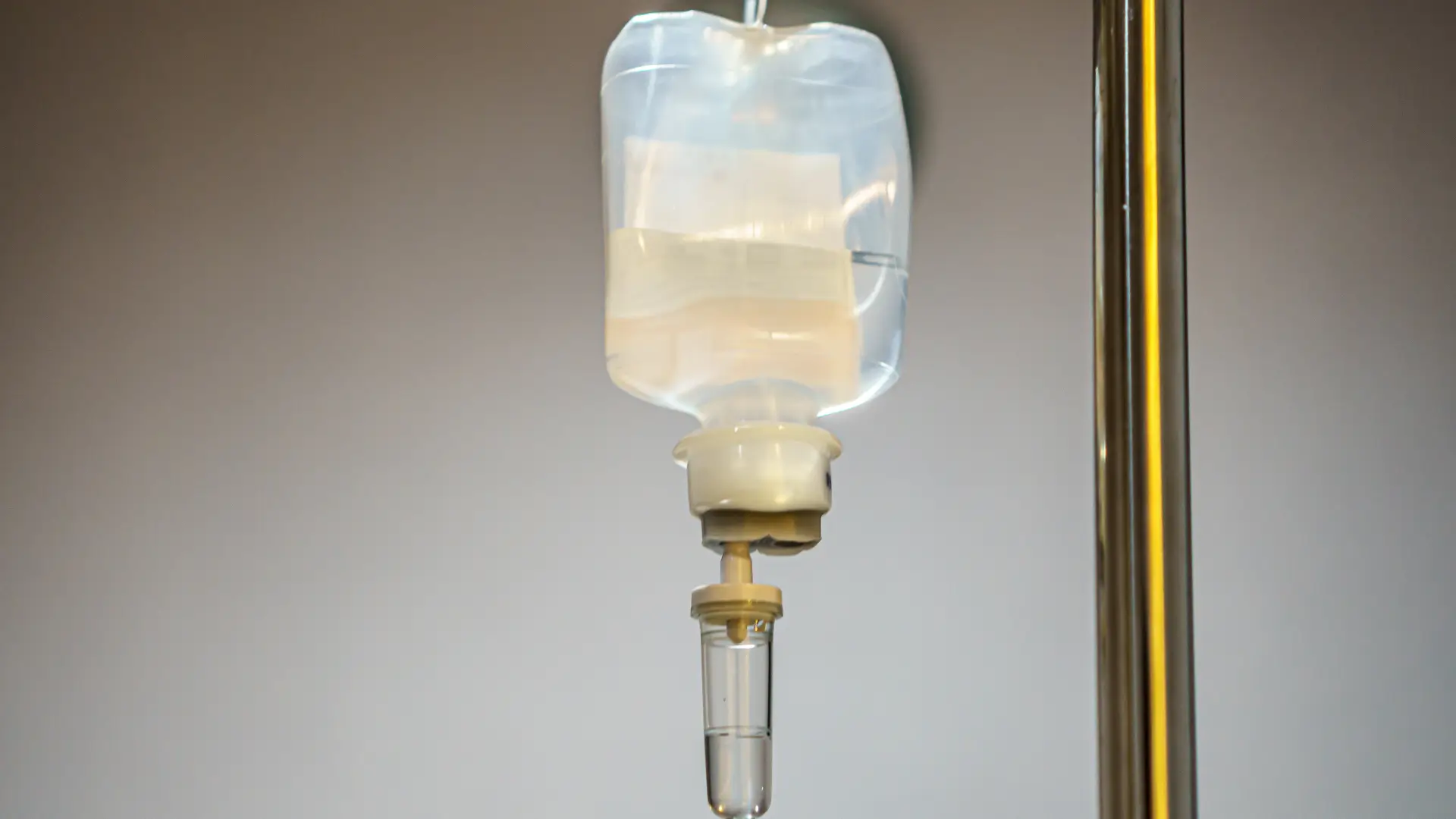
A 2020 study published in Frontiers in Pharmacology explored the variables to consider when prioritizing drugs for precision dosing, emphasizing that standard, fixed dosing regimens may be suboptimal. The review highlighted factors such as a drug’s therapeutic index, pharmacokinetic/pharmacodynamic variability, and disease-state characteristics to assess the potential for individualized dosing to improve patient outcomes.
Building on this principle, proper dosing is equally essential in inflammatory bowel disease (IBD) management. Entyvio, a biologic therapy approved for moderate to severe ulcerative colitis and Crohn’s disease, may benefit from a precision dosing approach to optimize therapeutic benefits while minimizing potential risks. Consideration of individual patient factors is crucial for achieving the ideal balance between safety and effectiveness.
In this article, we will discuss the recommended dosing strategies for Entyvio, options for maintenance treatments, administration protocols, and management of missed doses.
Key Takeaways on Entyvio Pen and Intravenous Infusion Dose
- Entyvio (vedolizumab) is approved for adults with moderate to severe ulcerative colitis or Crohn’s disease, with a standard induction regimen of 300 mg IV at weeks 0, 2, and 6, followed by maintenance every 8 weeks.
- Self-injection options (108 mg) are available starting from week 14, allowing flexibility and potential for at-home administration.
- No routine dose adjustments are needed for older adults or those with mild to moderate renal/hepatic impairment, but close monitoring is advised.
- Entyvio is not approved for pediatric use, and pregnant patients require individualized risk assessment due to limited data.
- Healthcare providers must monitor for infusion reactions, infections, and potential drug interactions, especially with immunosuppressants and corticosteroids.
- For missed doses, advise patients to take it as soon as possible and resume the regular biweekly schedule without doubling doses.
- Regular follow-ups and adherence to administration protocols are essential to minimizing Entyvio side effects and maximizing therapeutic benefit.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Entyvio wholesale at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Entyvio’s Recommended Induction and Maintenance Dosing Schedules
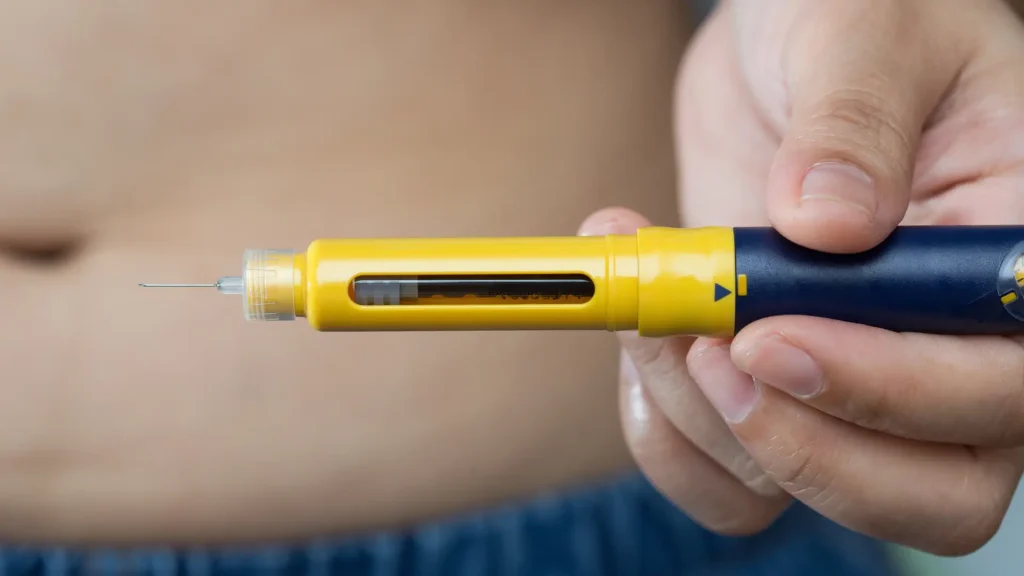
A healthcare provider may prescribe the U.S. Food and Drug Administration-approved Entyvio (vedolizumab) as treatment for adult individuals with moderate to severe ulcerative colitis or Crohn’s disease. These providers must adhere to the correct Entyvio dose and dosage schedule for optimal outcomes, maximizing therapeutic benefits while minimizing risks.
Furthermore, providers and patients may collaborate to design a personalized treatment plan for a more holistic approach. The infusion solution and Entyvio pen provide options for different patient needs and preferences.
Below are the recommended induction and maintenance regimens:
- Induction Phase: A healthcare provider must administer a recommended 300 mg Entyvio intravenous infusion at weeks 0, 2, and 6. Some dosages may be adjusted based on a patient’s specific condition or needed treatment protocol. Monitor patients closely during this period to assess therapeutic response and identify adverse reactions.
- Maintenance Phase: Entyvio offers two options for maintenance therapy: IV infusion or a 108 mg Entyvio subcutaneous injection via the Entyvio pen. Starting at week 14, patients can continue with 300 mg infusions every 8 weeks. Adjust dosing intervals based on the patient’s individual response and clinical evaluation.
The starter doses—also known as loading doses— during the induction phase allow the medicine to reach therapeutic levels in the body efficiently. The infusion must be performed using aseptic techniques, and the solution should be diluted and checked for clarity before administration.
Meanwhile, the maintenance dosage may vary depending on the patient’s clinical response. Healthcare providers must regularly assess treatment progress and adjust the dosage as needed. Healthcare providers should discontinue Entyvio if no therapeutic benefit is observed by week 14.
Entyvio Dosage Adjustments for Special Populations

While the standard dosage applies to most people, certain patients may require closer patient assessment and monitoring. The FDA-approved labeling does not specify routine dosing adjustments for special populations.
- Older People and Those with Organ Dysfunction: While they typically continue with the same standard dose, their health status warrants regular assessments by their healthcare provider.
- Pediatric and Pregnant Patients: Entyvio is only approved for adults. Providers must exercise caution and weigh the benefits and potential risks before starting treatment. No established dose adjustments exist for these groups, and the medicine should only be used when the potential benefit justifies any potential risk.
Administration Protocols of Entyvio: IV Infusion and Subcutaneous Injection
Entyvio can be given either by intravenous infusion or subcutaneous injection, depending on the prescribed maintenance treatment plan. Proper administration is crucial to ensure safety and therapeutic success.
Healthcare providers follow evidence-based protocols to administer medications and minimize Entyvio side effects:
- IV Infusion: Use aseptic techniques, verify dosage accuracy, and monitor for infusion-related reactions. Prepare the infusion solution by carefully reconstituting and diluting the vial contents under aseptic conditions. The Entyvio intravenous infusion typically lasts about 30 minutes. During and after infusion, patients must be monitored for hypersensitivity or infusion-related reactions.
- Subcutaneous Injection: Administer subcutaneous Entyvio via abdomen or thigh, following proper cleaning and injection angle protocols. Patients should rotate injection sites to prevent irritation. Before use, they should inspect the solution to ensure it is clear and colorless.
Co-administration of Entyvio with Other Therapies and Drug Dosage Interactions
Healthcare providers must have comprehensive knowledge about Entyvio to ensure patient safety throughout the treatment. This includes adhering to the recommended administration techniques and treatment protocols and understanding the potential drug interactions.
When used alongside other therapies, this treatment for inflammatory bowel disease requires careful coordination. A healthcare provider assesses potential drug interactions and makes necessary adjustments to ensure patient safety and optimize outcomes. For instance:
- Immunosuppressants: May increase infection risk—monitor closely.
- Biologics or Corticosteroids: Co-use requires evaluation to avoid efficacy interference or adverse events.
Monitoring and Managing Missed Doses of Entyvio

Clinicians perform regular evaluations to track therapeutic responses and manage side effects. While standard dosing generally applies, adjustments may be made based on patient outcomes and emerging clinical evidence to optimize treatment. Moreover, patients may also miss some doses of their Entyvio treatment.
The most common and essential tips for missed subcutaneous injections of Entyvio include the following:
- Take missed doses as soon as remembered, then resume biweekly dosing.
- Do not take double doses.
- Encourage patient communication if uncertain.
Providers must underscore the importance of adhering to prescribed schedules and educating patients on handling missed doses. Regular follow-ups with healthcare providers help assess the treatment’s effectiveness and allow for adjustments if needed. This proactive approach minimizes potential side effects and ensures the best possible outcomes.
Conclusion
Understanding the dosing protocols for Entyvio is essential for healthcare providers managing patients with moderate to severe inflammatory bowel disease. Following the recommended induction and maintenance regimens, practitioners can personalize treatment plans that optimize therapeutic benefits while minimizing potential side effects.
Moreover, attention to special populations, such as older patients and those with organ dysfunction, can enhance safety and treatment outcomes. Adhering to administration guidelines and recognizing drug interactions, healthcare providers play a pivotal role in improving patient care and ensuring the best possible results with Entyvio therapy.
FAQs
1. What is the recommended induction dosing schedule for Entyvio?
The recommended induction regimen for Entyvio involves administering 300 mg intravenously at weeks 0, 2, and 6. Healthcare providers should closely monitor patient responses and any potential side effects during this period.
2. How often should maintenance doses of Entyvio be administered?
For maintenance therapy, patients can receive 300 mg infusions every 8 weeks or opt for self-injection using the Entyvio pen (108 mg) starting at week 14, depending on what is best for their individual treatment plan.
3. Are there any special considerations for older patients or those with organ dysfunction regarding Entyvio dosing?
Standard dosing is generally applied for older patients and those with mild to moderate renal or hepatic impairment. However, healthcare providers should closely monitor these patients to ensure the treatment remains safe and effective without needing dose modifications.
References
- Tyson RJ, Park CC, Powell JR, et al. Precision dosing Priority criteria: drug, disease, and patient population variables. Frontiers in Pharmacology. 2020;11. doi:10.3389/fphar.2020.00420
- Infusions and Subcutaneous Injections | ENTYVIO® (vedolizumab). ENTYVIO. https://www.entyvio.com/administration
- Seabright J. Dosage details for entyvio. Healthline. Published March 7, 2025. https://www.healthline.com/health/drugs/entyvio-dosage