Tocilizumab, the generic name for Actemra, has garnered significant attention in the medical field due to its effectiveness in treating autoimmune disorders. A recent phase 3, randomized, double-blind study, published in 2024 in Arthritis Research & Therapy, demonstrated the impressive results of tocilizumab for managing moderate-to-severe rheumatoid arthritis (RA). In this study, more than 87% of patients achieved an ACR20 response by week 48, underscoring the drug’s ability to significantly reduce disease activity.
Building upon these results, Actemra (tocilizumab) offers a targeted therapeutic approach for autoimmune diseases. By modulating the interleukin-6 (IL-6) pathway, this medication helps reduce systemic inflammation, leading to improved patient outcomes. Actemra’s formulation continues to provide physicians with a refined treatment strategy for managing inflammatory conditions, further solidifying its role in modern medicine.
In this article, we will explore Actemra’s generic name (tocilizumab), examine its therapeutic mechanism, and discuss its role within the evolving landscape of biologic therapies for rheumatoid arthritis.
Key Takeaways
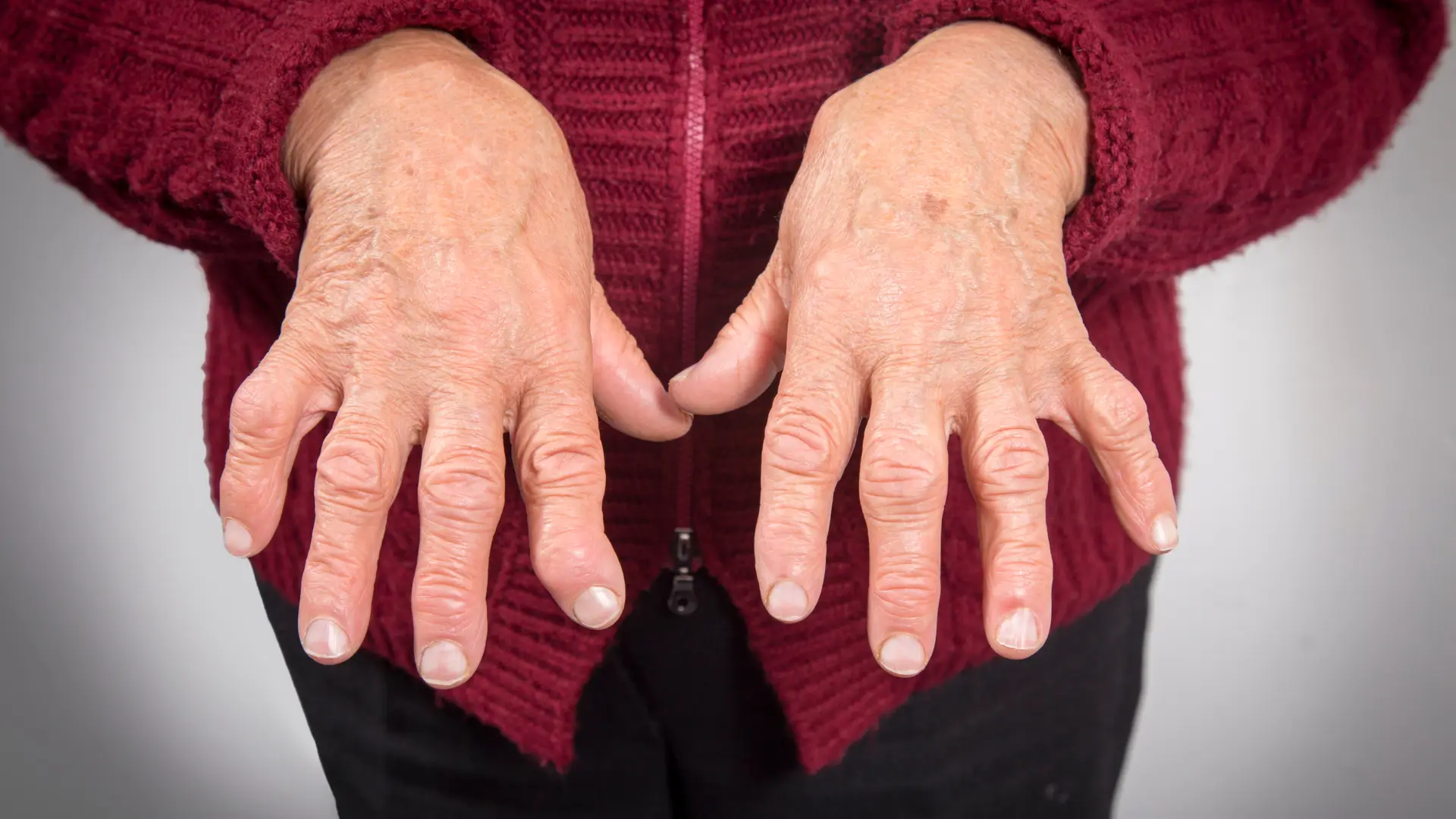
- Tocilizumab (Actemra) is a monoclonal antibody that targets interleukin-6 (IL-6) receptors to reduce inflammation and manage various autoimmune conditions, such as rheumatoid arthritis and giant cell arteritis.
- It blocks the IL-6 receptor, inhibiting pro-inflammatory cytokine release, which helps alleviate symptoms of inflammatory diseases and prevents further tissue damage.
- FDA-approved for several conditions, including rheumatoid arthritis, juvenile idiopathic arthritis, and cytokine release syndrome, with ongoing studies exploring off-label uses of tocilizumab.
- Tocilizumab dosing varies depending on the patient’s condition, with both intravenous (IV) and subcutaneous (SC) formulations available. Dosing adjustments are made based on weight, clinical response, and comorbidities.
- Common side effects include upper respiratory tract infections, headaches, and hypertension. Serious risks, like pancreatitis and liver issues, require close monitoring.
- Biosimilars of Actemra, such as Avtozma and Tofidence, offer more affordable treatment options while maintaining similar efficacy and safety profiles.
- Pre-treatment screening is crucial to ensure patient suitability for tocilizumab, including tuberculosis screening and liver function tests.
- Patient monitoring during treatment is essential to detect any adverse reactions early and ensure the safety and effectiveness of the tocilizumab therapy.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Actemra online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Pharmacological Profile of Tocilizumab
Tocilizumab is a humanized monoclonal antibody belonging to the IgG1 subtype, designed using recombinant DNA technology to minimize immunogenicity while maintaining a high affinity for IL-6 receptors. This advanced engineering makes it a targeted immunomodulatory therapy for various inflammatory conditions.
It binds specifically to soluble and membrane-bound IL-6 receptors, effectively blocking interleukin-6 from triggering pro-inflammatory signaling. This selective inhibition sets it apart from other biologics that target different cytokine pathways, ensuring precise effects on immune modulation.
Furthermore, by blocking IL-6 receptor activity, tocilizumab disrupts the JAK/STAT signaling cascade, reducing acute-phase proteins and pro-inflammatory cytokines. This suppression alleviates systemic inflammation, benefiting patients with autoimmune diseases like rheumatoid arthritis.
The reduction of IL-6 activity enables patients taking Actemra (tocilizumab) to slow disease progression and often allows physicians to taper corticosteroid use, minimizing side effects. This mechanism, drugs tocilizumab reinforces its role as a cornerstone therapy in managing chronic inflammatory and autoimmune conditions.
Drug Information: Clinical Indications for Tocilizumab

The US Food and Drug Administration (FDA) has approved tocilizumab for treating rheumatoid arthritis, where it effectively reduces joint inflammation and enhances functional mobility. Its label states that it also plays a crucial role in managing giant cell arteritis by targeting vascular inflammation and minimizing corticosteroid dependence.
Other approved indications of this specific tocilizumab include polyarticular juvenile idiopathic arthritis and systemic juvenile idiopathic arthritis in patients aged two years and older. Furthermore, it remains the only FDA-approved therapy for cytokine release syndrome in adult and pediatric patients.
On the other hand, several studies suggest that tocilizumab may have off-label applications in other autoimmune diseases. A study had explored its potential in non-juvenile idiopathic arthritis in pediatric rheumatic patients, where IL-6 plays a significant role in the treatment.
However, each health care provider must carefully evaluate emerging evidence, balancing therapeutic benefits against patient-specific risks while closely monitoring treatment outcomes. As ongoing studies expand to tocilizumab‘s clinical utility and effects, its off-label applications may become viable treatment options pending further regulatory review and approval.
Availability and Biosimilars of Tocilizumab

While understanding tocilizumab, patients may ask, “What is Actemra?” The FDA has approved this prescription medication as an interleukin-6 (IL-6) receptor antagonist. The health care provider can use the official drug information of Actemra (tocilizumab) to treat the following concerns:
- Moderate to Severe Rheumatoid Arthritis
- Systemic Sclerosis with Early Interstitial Lung Disease
- Giant Cell Arteritis
- Polyarticular Juvenile Idiopathic Arthritis
- Systemic Juvenile Idiopathic Arthritis
- Coronavirus Disease 2019
The brand has conducted rigorous clinical trials to prove its safety and efficacy in addressing these medical conditions. These indications highlight Actemra’s versatility in suppressing IL–6–mediated inflammation.
Additionally, several biosimilars have emerged as approved alternatives to Actemra, reflecting rigorous clinical and analytical evaluations that confirm their high structural and functional similarity. These include:
- Avtozma (tocilizumab-anoh)
- Tofidence (tocilizumab-bavi)
- Tyenne (tocilizumab-aazg)
These biosimilars offer additional treatment choices, promote market competition, and have the potential to lower therapy costs while maintaining comparable efficacy, safety, and immunogenicity profiles.
Considerations for Prescribing Tocilizumab (Actemra) of A Care Provider

Before taking Actemra, a provider must determine patient eligibility for tocilizumab therapy based on confirmed indications such as moderately to severely active rheumatoid arthritis, giant cell arteritis, and cytokine release syndrome. A comprehensive medical history review, including infection risks, liver disease, and previous malignancies, ensures that only suitable candidates receive treatment, optimizing therapeutic outcomes.
Pre-treatment assessments for tocilizumab include:
- Tuberculosis screening
- Liver function tests
- Complete blood counts
They should also evaluate contraindications, such as hypersensitivity to tocilizumab or active infections, ensuring a safe and effective treatment initiation.
Dosing and Monitoring Considerations
Actemra (tocilizumab) dosing follows established clinical guidelines and label:
- Intravenous administration typically starts at 4 mg/kg, with an option to increase to 8 mg/kg based on patient response.
- Subcutaneous therapy uses fixed doses, adjusted according to patient weight, ensuring consistent drug exposure and effective disease management.
Regular clinical and laboratory monitoring is essential throughout tocilizumab therapy. Ongoing drug information highlights the importance of regular monitoring. Clinical evaluations complement laboratory tests, allowing providers to monitor effects for signs of infection, injection site reactions, and systemic symptoms. They can adjust doses promptly if adverse effects arise and ensure patient safety through proactive intervention.
Potential Side Effects
Getting treated with Actemra may cause common side effects, including upper respiratory tract infections, headache, hypertension, or injection site reactions. These Actemra common reactions may resolve with minimal intervention within a few days to a week.
Additionally, Tocilizumab may cause serious infections, elevated liver enzymes, neutropenia, and lipid abnormalities. Patients are advised to report symptoms such as fever, respiratory issues, or unusual discomfort immediately. Key Actemra side effects or adverse events include:
- Serious Infections and Latent Tuberculosis Reactivation
- Serious Allergic Reactions
- Nervous System Problems
The drug information stresses that regular monitoring with a health care provider reduces risks and ensures safe, long-term use. By carefully following the FDA-approved label of tocilizumab and working closely with a care provider, patients can achieve strong clinical outcomes while minimizing treatment-related risks.
Conclusion
Tocilizumab, known by its brand name Actemra, plays a crucial role in treating various inflammatory conditions, particularly moderate-to-severe rheumatoid arthritis and giant cell arteritis. Its ability to specifically inhibit interleukin-6 signaling significantly reduces systemic inflammation, improving patient outcomes and mobility. The FDA’s approval for multiple indications solidifies its importance in modern therapeutic strategies.
As research continues to evolve, healthcare professionals and patients must stay informed about the latest findings and potential off-label uses or effects of tocilizumab. Ongoing education and awareness will help optimize treatment approaches and enhance the understanding of this vital medication in managing autoimmune diseases.
FAQs
1. What is tocilizumab, and what is its brand name?
Tocilizumab is a humanized monoclonal antibody that acts as an interleukin-6 receptor antagonist. It is commonly known by its brand name, Actemra.
2. What conditions is tocilizumab (Actemra) approved to treat?
The FDA approves Tocilizumab for health concerns, such as moderate to severe rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
3. Are there any biosimilars available for tocilizumab?
Yes, several biosimilars of tocilizumab, such as Avtozma, Tofidence, and Tyenne, have been approved as alternatives to Actemra. They may have a different label but these offer similar efficacy and safety profiles.
References
- Leng X, Leszczyński P, Jeka S, et al. A phase 3, randomized, double-blind, active-controlled clinical trial to compare BAT1806/BIIB800, a tocilizumab biosimilar, with tocilizumab reference product in participants with moderate-to-severe rheumatoid arthritis with inadequate response to methotrexate: treatment period 2 analysis (week 24 to week 48). Arthritis research & therapy. 2024;26(1):157. doi:https://doi.org/10.1186/s13075-024-03375-w
- Jung JY, Kim MY, Suh CH, Kim HA. Off-label use of tocilizumab to treat non-juvenile idiopathic arthritis in pediatric rheumatic patients: a literature review. Pediatr Rheumatol Online J. 2018;16(1):79. Published 2018 Dec 14. doi:10.1186/s12969-018-0296-z