A recent study published in Lupus Science & Medicine (2024) highlighted that the majority of patients with systemic lupus erythematosus (SLE) who received intravenous immunoglobulin (IVIG) treatment experienced mild adverse effects, including myalgia and transient fever. These findings underscore the efficacy and tolerability of intravenous therapies, but they also emphasize the importance of recognizing and managing side effects.
Similarly, innovative treatments like Saphnelo have shown promising results in SLE management, yet, like any therapy, they come with the potential for side effects. Understanding and closely monitoring these reactions is critical to ensuring both patient safety and optimal therapeutic outcomes.
In this article, we will present a comprehensive list of Saphnelo’s side effects, providing essential insights for both healthcare providers and patients to make informed, confident decisions about treatment options.
Key Takeaways
- Saphnelo (anifrolumab-fnia) is an FDA-approved treatment for systemic lupus erythematosus (SLE), offering relief for patients with moderate to severe disease.
- Common side effects of Saphnelo (occurring in ≥5% of patients) include upper respiratory infections, bronchitis, infusion reactions (fever, chills, dizziness), shingles, and cough. These side effects are typically mild to moderate but require monitoring.
- While serious adverse reactions have a low chance of happening, they may include severe infections, anaphylaxis, increased risk of malignancies, and exacerbation of pre-existing conditions.
- Infusion-related reactions such as fever, chills, and rash can occur during or after the intravenous infusion. Slowing the infusion rate or pausing the treatment may help alleviate mild symptoms. However, severe reactions require urgent medical attention.
- To mitigate risks, clinicians should use premedication, adjust infusion rates as needed, and educate patients on recognizing early signs of adverse reactions. Routine monitoring of clinical responses and laboratory markers is crucial for ensuring safety.
- Regular follow-up visits are crucial for assessing treatment efficacy, detecting infections early, and ensuring overall patient well-being during Saphnelo therapy.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Saphnelo online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Common Side Effects of Saphnelo (≥5%)

Saphnelo (anifrolumab-fnia) is an intravenous monoclonal antibody approved for treating moderate to severe systemic lupus erythematosus (SLE). While Saphnelo’s FDA approval assures its safety and effectiveness, like all treatments, it may still cause side effects.
According to the manufacturer, several common Saphnelo side effects affect at least 5% of patients. Understanding these reactions is crucial for medical professionals to manage therapy properly and ensure patient safety. The most frequently reported adverse events include:
- Upper Respiratory Infections: These may manifest as nasal congestion, a sore throat, or sinus discomfort.
- Bronchitis: Symptoms include cough, chest tightness, and mucus production.
- Infusion Reactions: Fever, chills, dizziness, and rash are common symptoms during or shortly after the infusion.
- Shingles (Herpes Zoster): Typically presents as painful blisters, usually on one side of the body.
- Cough: Can be either dry or productive, sometimes with throat irritation.
These typical but unwanted effects are usually mild to moderate, but still require careful monitoring. Patients should report side effects that persist or worsen to their healthcare provider to prevent complications.
Serious & Rare Adverse Reactions to Saphnelo
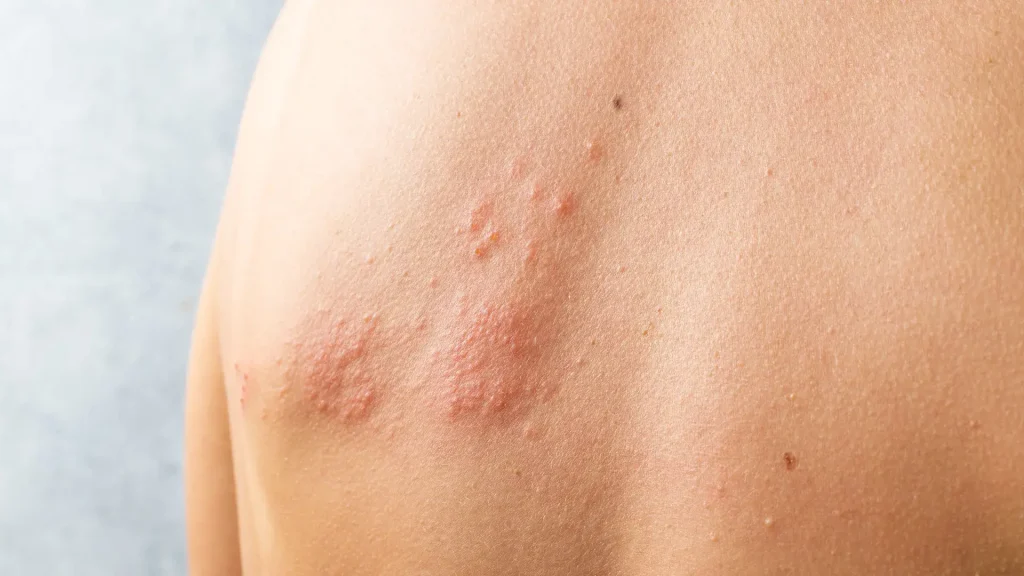
Although Saphnelo is FDA-approved, it may trigger rare yet serious side effects. These events are infrequent but can have a significant impact on patient management and safety during treatment.
- Immune System Suppression: Saphnelo may suppress the immune system, increasing the risk of severe infections, such as respiratory infections, pneumonia, and shingles. These infections could lead to hospitalization and require immediate adjustments to the treatment plan.
- Hypersensitivity Reactions: Rare anaphylactic reactions, such as swelling in the face, tongue, or throat, along with trouble breathing, have been reported. Immediate medical attention is necessary for proper management.
- Increased Risk of Malignancies: Reduced immune surveillance could lead to an increased risk of cancers in some patients.
- Exacerbation of Pre-existing Conditions: As an immune-modulating therapy, Saphnelo may worsen conditions related to immune modulation, requiring careful monitoring.
- Severe Infusion Reactions: Some patients experience severe reactions during the infusion of drugs that require urgent medical intervention.
Healthcare professionals should carefully monitor patients for these drug-related side effects and adjust treatment promptly. Any new or unexpected symptoms should be reported promptly, following Saphnelo’s official prescribing information.
Infusion‑Related & Hypersensitivity Events with Saphnelo
Saphnelo administration requires an intravenous infusion over 30 minutes. While most patients tolerate the treatment well, some may experience infusion-related or hypersensitivity reactions. Early detection and proper management are crucial to maintaining patient safety.
Infusion-related reactions might include fever, chills, nausea, dizziness, and rash, typically occurring during or shortly after the infusion. When mild symptoms arise, the infusion rate can be slowed or temporarily paused to alleviate discomfort. However, in cases of severe reactions, immediate medical intervention is required.
In addition, hypersensitivity reactions, including anaphylaxis that may lead to trouble breathing, have been reported. These drug reactions necessitate urgent care and proper symptom management to ensure the patient’s safety.
Risk Mitigation & Patient Monitoring for Saphnelo
Effective risk mitigation and vigilant patient monitoring are essential for ensuring safe and effective treatment with Saphnelo (anifrolumab-fnia) in systemic lupus erythematosus. Clinicians must adhere to the protocols outlined in Saphnelo’s official prescribing information to maintain the highest standards of safety.
Several key risk mitigation strategies include:
- Premedication: Administering premedications to manage infusion-related reactions and reduce discomfort and unwanted effects during treatment.
- Infusion Rate Adjustments: Slowing down the infusion rate when necessary to minimize adverse reactions.
- Patient Education: Educating patients on how to recognize early signs of adverse reactions, allowing for prompt response and treatment.
Additionally, a healthcare provider should routinely assess each patient’s clinical response and monitor key laboratory markers during therapy. Regular follow-up visits are crucial for evaluating treatment efficacy, detecting infections at an early stage, assess emerging drug interactions, and ensuring overall patient well-being. Proactive monitoring enables timely interventions, improving patient outcomes and maintaining Saphnelo’s safety profile.
By following these strategies and closely monitoring patients, healthcare professionals can optimize the effectiveness of anifrolumab while ensuring patient safety throughout the treatment process.
Conclusion
Saphnelo (anifrolumab-fnia) offers a promising treatment option for systemic lupus erythematosus, providing relief for patients with moderate to severe disease. While the FDA approval of Saphnelo ensures its safety and efficacy, it’s vital to be aware of both common and rare side effects that may occur. Understanding these reactions is key to effectively managing treatment and ensuring the well-being of patients.
Healthcare providers must closely monitor patients during and after Saphnelo infusions, as both mild and serious adverse effects can arise. By implementing risk mitigation strategies and educating patients about potential side effects, clinicians can optimize therapeutic outcomes and improve overall patient care.
FAQs
1. What are the common side effects of Saphnelo?
Common Saphnelo side effects include upper respiratory infections, bronchitis, infusion reactions, shingles, and cough, affecting at least 5% of patients.
2. Are there serious side effects associated with Saphnelo?
Yes, serious side effects can occur when using Saphnelo. These include severe infections, anaphylaxis, and exacerbation of pre-existing conditions. Patients should seek immediate medical attention for any severe reactions that occur.
3. How can healthcare providers manage infusion-related reactions with Saphnelo?
Providers can manage infusion-related reactions by adjusting the infusion rate, administering pre-medication, and closely monitoring patients for any adverse symptoms during and after treatment.
References
- Nur Kaya M, Kılıç Ö, Canbaş M, Sungur Özgünen M, Çimen Güneş E, Yılmaz S. Role of intravenous immunoglobulins in the management of systemic lupus erythematosus: a single-centre experience. https://lupus.bmj.com/content/lupusscimed/11/2/e001402.full.pdf.
- US-92602 HIGHLIGHTS OF PRESCRIBING INFORMATION | SAPHNELO®. SAPHNELO. Accessed June 16, 2025. https://drd9vrdh9yh09.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/44b6985c-8268-46b1-ba3e-2bb43bfd4d4c/44b6985c-8268-46b1-ba3e-2bb43bfd4d4c_viewable_rendition__v.pdf