According to the International Osteoporosis Foundation, approximately one in three women over the age of 50 will experience osteoporosis-related fractures, highlighting the importance of effective treatment options for postmenopausal women.
While many individuals suffer from these bone concerns in their older ages, it urges them to seek potent options to manage their condition. Among many options, Prolia (denosumab) is a prescription medication used to treat osteoporosis and prevent bone loss. It works by inhibiting the breakdown of bone, helping to increase bone mass and strength.
In this article, we will explore the potential Prolia side effects, including its common and rare side effects, and how to manage and prevent these reactions.
Key Takeaways
- Prolia is a prescription medication for osteoporosis that helps increase bone mass but comes with a range of potential side effects.
- The side effects of Prolia are categorized into common, mild reactions and rare, serious complications that can arise during treatment.
- Frequent monitoring is essential to identify adverse reactions early, ensuring patients remain safe throughout their Prolia treatment.
- Implementing strategies such as regular dental check-ups and monitoring calcium levels can effectively mitigate the risks associated with Prolia side effects.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Our sales representatives can advise you on how to buy Prolia online today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Common and Mild Prolia Side Effects

The Prolia manufacturer, Amgen, has developed this FDA-approved prescription medication to address various bone health concerns. It is indicated for:
- Postmenopausal Osteoporosis: Reducing fracture risk in women at high risk for fractures, including those with glucocorticoid-induced osteoporosis.
- Male Osteoporosis: Increasing bone mass in men at high risk for fractures, including those on androgen deprivation therapy for nonmetastatic prostate cancer.
- Bone Loss In Breast Cancer Patients: Supporting women receiving adjuvant aromatase inhibitor therapy who are at high risk for fractures.
Although Prolia has a well-established safety and efficacy profile, healthcare providers and patients should be aware of common side effects. Understanding these helps ensure informed decision-making and proper management.
Commonly reported side effects include
- Postmenopausal Osteoporosis: Back pain, limb pain, high cholesterol, muscle pain, and bladder infections.
- Male Osteoporosis: Back pain, joint pain, and cold symptoms (runny nose or sore throat).
- Corticosteroid-Induced Osteoporosis: Back pain, high blood pressure, bronchitis, and headaches.
- Cancer Treatment-Related Bone Loss: Joint pain, back pain, limb pain, and muscle discomfort.
To manage mild reactions effectively, practitioners may recommend:
- Using heat or ice packs for muscle pain relief.
- Engaging in gentle stretches and movement to ease discomfort.
- Avoiding irritation at the injection site by not scratching or rubbing.
- Staying hydrated and ensuring adequate rest.
- Taking over-the-counter pain relievers if necessary.
Serious and Rare Adverse Reactions to Prolia
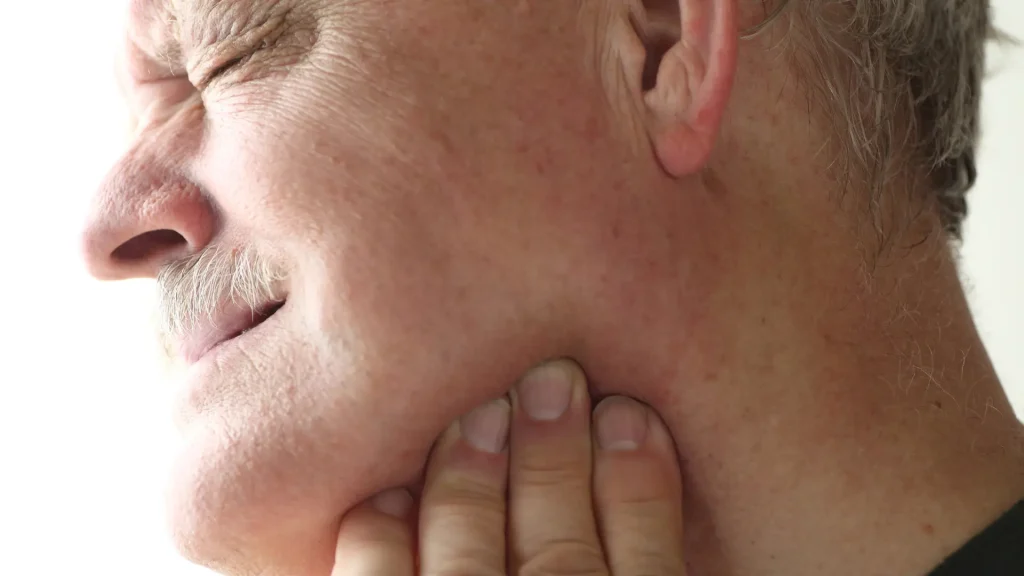
While Prolia has a favorable safety profile, some patients may experience rare but serious side effects. These reactions may result from individual treatment responses, improper administration, unsterile conditions, or underlying contraindications.
- Osteonecrosis Of The Jaw (Onj): A dental exam is essential before starting Prolia to assess risk. Maintaining good oral hygiene throughout treatment helps prevent complications.
- Atypical Femur Fractures: Some patients may develop unusual thigh bone fractures, often presenting as new or unexplained pain in the hip, groin, or thigh.
- Hypocalcemia: Prolia can lower calcium levels, which must be corrected before treatment begins to avoid serious complications.
Risk Factors and Warning Signs
Patients at higher risk for severe complications include those with prolonged Prolia use, pre-existing dental issues, or a history of bisphosphonate therapy. Warning signs include:
- Persistent jaw pain (potential ONJ)
- Unexplained hip, groin, or thigh pain (possible atypical fracture)
- Symptoms of hypocalcemia, such as muscle cramps, numbness, or tingling
To ensure patient safety, practitioners should implement regular clinical monitoring, including:
- Routine dental check-ups to detect early signs of ONJ.
- Serum calcium level assessments to prevent hypocalcemia-related complications.
- Monitoring for fractures or jaw issues to ensure early intervention.
If severe reactions occur, treatment strategies may include discontinuing Prolia, correcting calcium levels, and seeking specialized dental or orthopedic care.
Managing and Preventing Prolia Side Effects

Medical professionals must employ the best practices throughout the Prolia treatment to maximize the medicine’s efficacy. Adhering to the indications provided in the Prolia prescribing information, practitioners can ensure proper patient selection. The counseling should involve informing and educating patients about the potential Prolia side effects, allowing them to make informed treatment decisions.
Additionally, ensuring patient safety with Prolia requires following the essential monitoring protocols and preventive measures. Medical professionals must understand these key points:
- Regular Laboratory Tests: Monitor calcium levels, renal function, and complete blood counts to detect abnormalities early.
- Oral Hygiene: Maintain good oral hygiene and schedule regular dental visits to prevent osteonecrosis of the jaw.
- Calcium and Vitamin D Intake: Ensure adequate calcium and vitamin D intake to prevent hypocalcemia.
- Symptom Education: Educate patients on recognizing symptoms of serious side effects, such as severe pain in the thigh, groin, or hip, and infections.
They may advise patients to seek medical attention promptly when reactions or symptoms persist or worsen. By following these best practices, healthcare providers can help minimize the risks associated with Prolia.
Conclusion
Prolia remains a valuable treatment option for osteoporosis, offering effective bone protection while carrying potential side effects of varying severity. While most side effects are manageable, being aware of rare but serious risks is essential for informed decision-making.
Empowering patients with knowledge about their treatment fosters confidence and a proactive approach to bone health, ensuring they can navigate their osteoporosis journey with clarity and reassurance.
FAQs
1. What are the common side effects of Prolia?
Common side effects of Prolia include back pain, joint pain, muscle pain, high cholesterol, and bladder infections for postmenopausal women. Men with osteoporosis may experience back pain and common cold symptoms.
2. What serious side effects should I be aware of while taking Prolia?
Serious side effects of Prolia include osteonecrosis of the jaw, atypical femur fractures, and hypocalcemia (low calcium levels). It’s essential to monitor for persistent jaw pain, new hip or thigh pain, and symptoms like muscle cramps and numbness.
3. How can patients manage mild side effects from Prolia?
Patients can apply heat or ice to painful areas, gently stretch, stay hydrated, and rest to manage mild side effects. Over-the-counter pain relievers may also be helpful. Always follow your healthcare provider’s guidance.
References
- International Osteoporosis Foundation. (n.d.). Epidemiology of osteoporosis and fragility fractures | International Osteoporosis Foundation. Www.osteoporosis.foundation. Retrieved February 18, 2025, from https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures
- HIGHLIGHTS OF PRESCRIBING INFORMATION Prolia ® (denosumab). (n.d.). Retrieved February 18, 2025, from https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Prolia/prolia_pi.pdf