Did you know the global market for intravenous (IV) hydration therapy was worth about USD 2.64 billion in 2024? Analysts expect it to nearly double by 2033, showing just how quickly IV treatments are expanding from hospitals into wellness clinics and concierge services.
Among the growing options, NAD IV therapy has captured particular interest. This treatment delivers nicotinamide adenine dinucleotide (NAD⁺)—a natural coenzyme vital for cellular energy production—directly into the bloodstream. Supporters say it may help boost cognitive function, speed up recovery after exercise, and enhance overall vitality, though research is still ongoing to confirm these effects.
In this article, we’ll break down how NAD IV therapy works, what the current science and patient experiences say about its benefits and risks, how it is commonly administered, and what both healthcare providers and patients should consider when deciding if it fits into a broader wellness plan.
Key Takeaways
- NAD IV therapy delivers nicotinamide adenine dinucleotide (NAD⁺) directly into the bloodstream to support energy production, DNA repair, and cellular signaling.
- While NAD⁺ levels can also be raised with oral precursors or intramuscular routes, IV infusion therapy delivery achieves higher plasma levels more rapidly.
- Proposed benefits include supporting DNA repair, helping regulate inflammation, and improving mitochondrial function, though most supporting evidence comes from preclinical or small pilot studies.
- Clinical indications such as chronic fatigue syndrome, fibromyalgia, and neurodegenerative conditions are off-label and investigational, since NAD IV therapy is not FDA-approved for these uses.
- Safe drip therapy administration requires trained providers, pharmaceutical-grade NAD⁺, aseptic technique, and continuous vital-sign monitoring during infusion.
- Typical dosing protocols vary (300–1,000 mg over 2–8 hours), with slower rates often improving patient comfort and reducing side effects.
- Reported side effects are usually mild, including headache, fatigue, or nausea, but long-term safety data are lacking.
- More large, placebo-controlled trials are needed to confirm efficacy, standardize dosing, and establish the true therapeutic role of NAD IV therapy.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Rejuvanad NAD IV, contact Medica Depot’s sales representatives and they will guide you on how to do so. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
What is NAD IV Therapy and How NAD+ Functions in the Body
NAD IV therapy refers to the intravenous delivery of nicotinamide adenine dinucleotide (NAD⁺), a coenzyme found in all living cells. NAD⁺ plays a central role in energy production, redox reactions, DNA repair, and cell signaling—processes that directly affect health and well-being.
While NAD⁺ levels can also be raised through oral precursors like nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN), as well as intranasal or intramuscular routes, IV infusion bypasses enzymatic conversion and raises plasma NAD⁺ more rapidly. This makes IV therapy appealing for those seeking higher, immediate bioavailability.
By supporting mitochondrial function, NAD⁺ helps convert glucose into usable cellular energy (ATP) and influences pathways involved in repair and resilience. Proposed benefits include:
- Supporting DNA repair through activation of PARP enzymes
- Helping regulate inflammatory responses via sirtuin activity
- Promoting mitochondrial efficiency and cellular defense against oxidative stress
It’s important to note that much of this evidence comes from preclinical or small human studies, and large clinical trials are still lacking. Maintaining optimal NAD⁺ levels is thought to contribute to healthier cellular aging and vitality, but these claims remain under active investigation.
Clinical Indications for NAD IV Therapy: When Medical Professionals Use It
Some practitioners use NAD IV therapy in wellness or integrative medicine settings to address conditions linked to reduced cellular energy and mitochondrial dysfunction. By infusing high-purity NAD⁺ directly into the bloodstream, intracellular coenzyme levels can be temporarily replenished, supporting metabolic and regenerative pathways.
Potential areas of interest, still considered off-label and investigational, include:
- Chronic Fatigue Syndrome and Fibromyalgia
- Neurodegenerative Disorders (e.g., Parkinson’s disease, Alzheimer’s disease)
- Mitochondrial myopathies and metabolic syndromes
However, NAD IV therapy is not FDA-approved (or EMA-approved) for these conditions. Most published studies are small, short-term, and exploratory, so use in these contexts is elective rather than standardized medical practice.
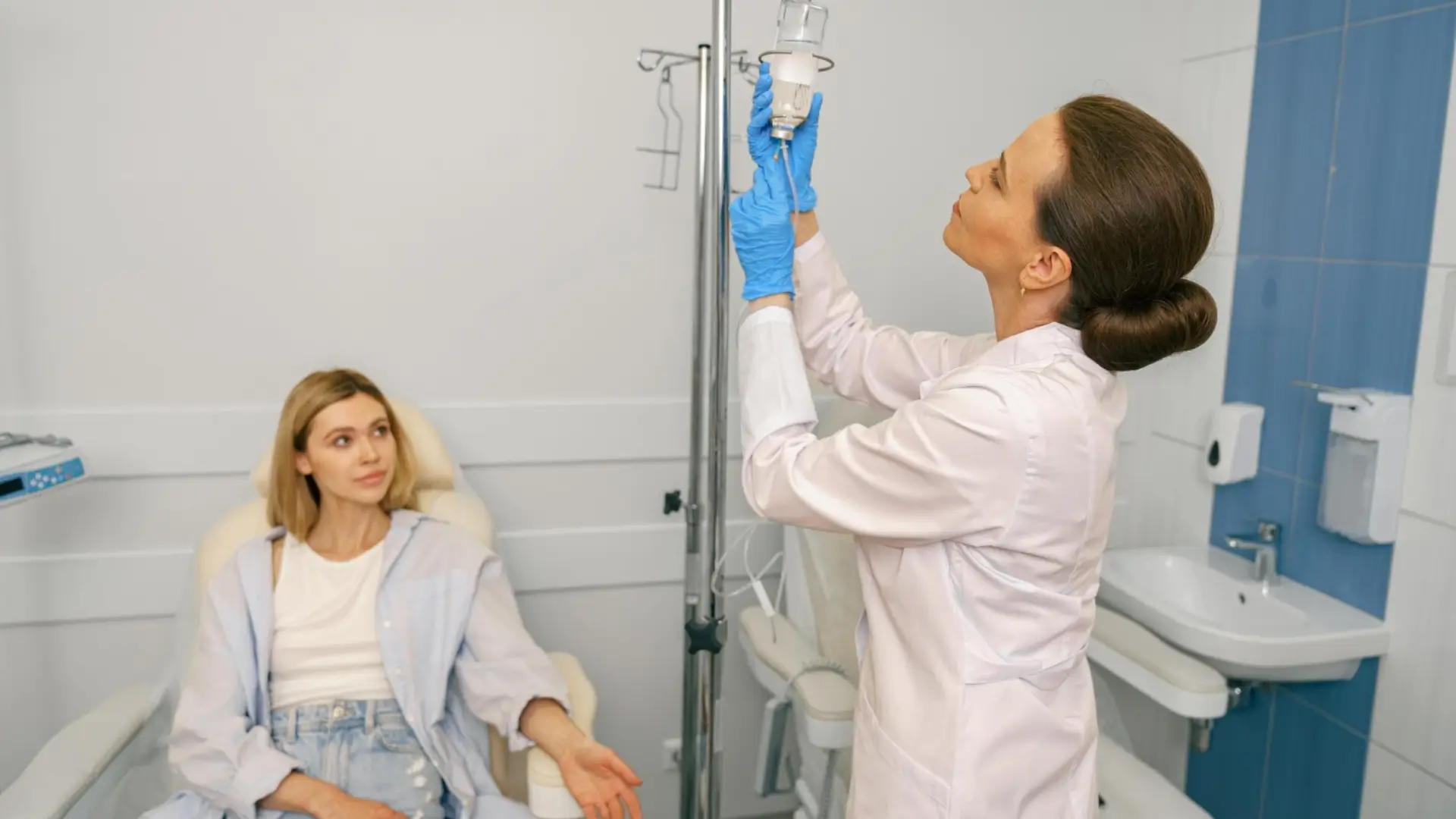
Protocols, Administration, and Monitoring of NAD IV Therapy
Before treatment, providers should understand what is in NAD IV therapy as well as how it is prepared, diluted, and infused. Training in IV administration and knowledge of safe NAD infusion protocols are necessary to minimize risks.
Typical practices include:
- Baseline Assessment: Document lab values, review medical history, and check for comorbidities.
- Sourcing: Use only pharmaceutical-grade NAD⁺, compounded under sterile conditions.
- Preparation: Draw the calculated NAD⁺ dose into a sterile IV bag with normal saline or dextrose solution, maintaining aseptic technique.
- Infusion: Use an infusion pump to deliver NAD⁺ slowly. Slower rates can reduce side effects such as flushing, chest tightness, or nausea.
- Monitoring: Check vital signs throughout the session. If intolerance occurs, pause or slow the infusion, then resume once symptoms resolve.
Post-Treatment Session
After IV drip infusion, clinicians typically:
- Record patient feedback on mood, energy, and cognitive clarity
- Recheck vital signs after a 30-minute observation
- Order follow-up lab tests to evaluate metabolic or organ function changes
Based on these findings, the treatment schedule may be continued, adjusted, or transitioned into a maintenance phase.
Evidence Base: Research, Gaps, and Quality of Studies on NAD IV Therapy

Early safety and pharmacokinetic trials suggest NAD⁺ IV therapy is generally well tolerated. In one small pilot study, participants who received 750 mg of NAD⁺ over six hours reported no infusion-related adverse events, and lab values remained stable.
Another randomized pilot trial compared NAD⁺ and NR (nicotinamide riboside) IV infusions in healthy volunteers. Both were safe, but NR produced higher measured NAD⁺ levels three hours after infusion.
Despite promising signals of NAD infusion therapy, significant evidence gaps remain:
- Small study sizes (often fewer than 50 participants) limit generalizability
- Open-label designs prevent placebo-controlled safety comparisons
- Short follow-up periods (hours to weeks) limit understanding of sustained effects
- Variable dosing protocols (300–1,000 mg, infused over 2–8 hours) prevent clear comparisons
Reported side effects in studies and clinical use include headache, fatigue, sleep disturbance, and mild gastrointestinal upset. Severe adverse events are rare, but long-term safety data are not yet established.
Further Clinical Research in the Future
To strengthen the scientific foundation for NAD IV therapy, future trials should:
- Include larger, placebo-controlled studies with consistent endpoints, such as NAD⁺ blood and tissue assays
- Extend follow-up duration to track safety and sustained benefits
- Enroll diverse populations with conditions like neurodegenerative or metabolic diseases
- Standardize dosing and infusion rates to clarify therapeutic windows
Conclusion
NAD IV therapy is an emerging option aimed at supporting cellular function, energy production, and overall well-being. By delivering NAD⁺ directly into the bloodstream, it may provide benefits for energy, recovery, and cognitive performance, though current evidence remains preliminary.
Its growing popularity highlights the importance of clear protocols, careful monitoring, and realistic patient counseling. Practitioners should emphasize that NAD IV therapy is not FDA-approved for treating specific conditions, and research is ongoing to determine its true clinical potential. For now, its role is best viewed as a supportive, investigational treatment within broader health and wellness plans.
FAQs
1. What is NAD IV therapy, and how does it work?
NAD IV therapy involves infusing nicotinamide adenine dinucleotide (NAD⁺) directly into the bloodstream. This coenzyme is central to energy production, DNA repair, and cellular communication.
2. What conditions can NAD IV therapy help treat?
NAD IV therapy is being studied for conditions such as chronic fatigue syndrome, fibromyalgia, and neurodegenerative disorders. However, this treatment is not FDA-approved for these uses, and more research is needed.
3. What should practitioners know before administering NAD IV therapy?
Practitioners should follow evidence-based protocols for dosing, NAD infusion therapy duration, and monitoring. They must assess each patient’s baseline health, use pharmaceutical-grade NAD⁺, and monitor carefully throughout the infusion.
References
Grand View Research. Intravenous (IV) Hydration Therapy Market [2023 Report]. www.grandviewresearch.com. Published 2023. Accessed September 19, 2025. https://www.grandviewresearch.com/industry-analysis/iv-hydration-therapy-market-report
Radenkovic D, Reason, Verdin E. Clinical Evidence for Targeting NAD Therapeutically. Pharmaceuticals (Basel). 2020;13(9):247. Published 2020 Sep 15. doi:10.3390/ph13090247