A pivotal study on Latisse revealed that participants experienced a 106% increase in eyelash fullness after 16 weeks, compared to just a 12% improvement in the placebo group. This significant contrast demonstrates the effectiveness of the prescription bimatoprost solution in enhancing eyelash appearance.
Latisse uses bimatoprost to deliver noticeable results, helping individuals achieve their desired eyelash goals with consistent use and adherence to the prescribed treatment protocol. However, potential side effects, such as eye color changes, highlight the importance of consulting with a healthcare professional before starting the treatment.
This article will delve into the risk of Latisse eye color changes, how bimatoprost affects eye pigmentation, and ways to manage this rare side effect.
Key Takeaways
- Latisse prolongs the anagen or growth phase of the eyelash hair cycle when applied.
- The bimatoprost in the Latisse solution may cause eye color changes due to the increasing melanin in the iris.
- As individuals may find this rare side effect concerning, it does not cause medical problems with the eyes.
- Unfortunately, postmarketing surveillance does not reliably show the frequency of Latisse eye color change side effect, and no further studies have targeted this rare symptom.
- A professional evaluation can help determine the cause of this change and offer appropriate management of the rare condition.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Latisse products, our dedicated sales agents can give you proper guidance. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Understanding Latisse and Its Active Ingredient

Latisse, developed by Allergan Aesthetics, is an FDA-approved medication designed to improve the appearance of eyelashes in individuals with hypotrichosis, a condition characterized by sparse or inadequate eyelashes. With consistent use, Latisse effectively enhances eyelash length, thickness, and darkness.
Latisse’s effectiveness lies in its active ingredient, bimatoprost, a synthetic prostaglandin analog initially used to treat glaucoma. Bimatoprost was found to promote eyelash growth as a side effect, leading Allergan to formulate Latisse specifically to harness this benefit for enhancing eyelashes.
When applied to the base of the upper eyelashes, Latisse prolongs the anagen (growth) phase of the hair cycle, stimulating increased hair growth. This mechanism helps to achieve longer, thicker, and darker lashes, providing a more defined and noticeable enhancement in eyelash appearance.
The Rare Side Effect

Despite its US FDA-approved use for inadequate eyelashes, patients must expect that common Latisse side effects may occur. Redness and itching typically happen but may resolve independently within a few days. Moreover, patients may also experience a rare side effect — eye color change.
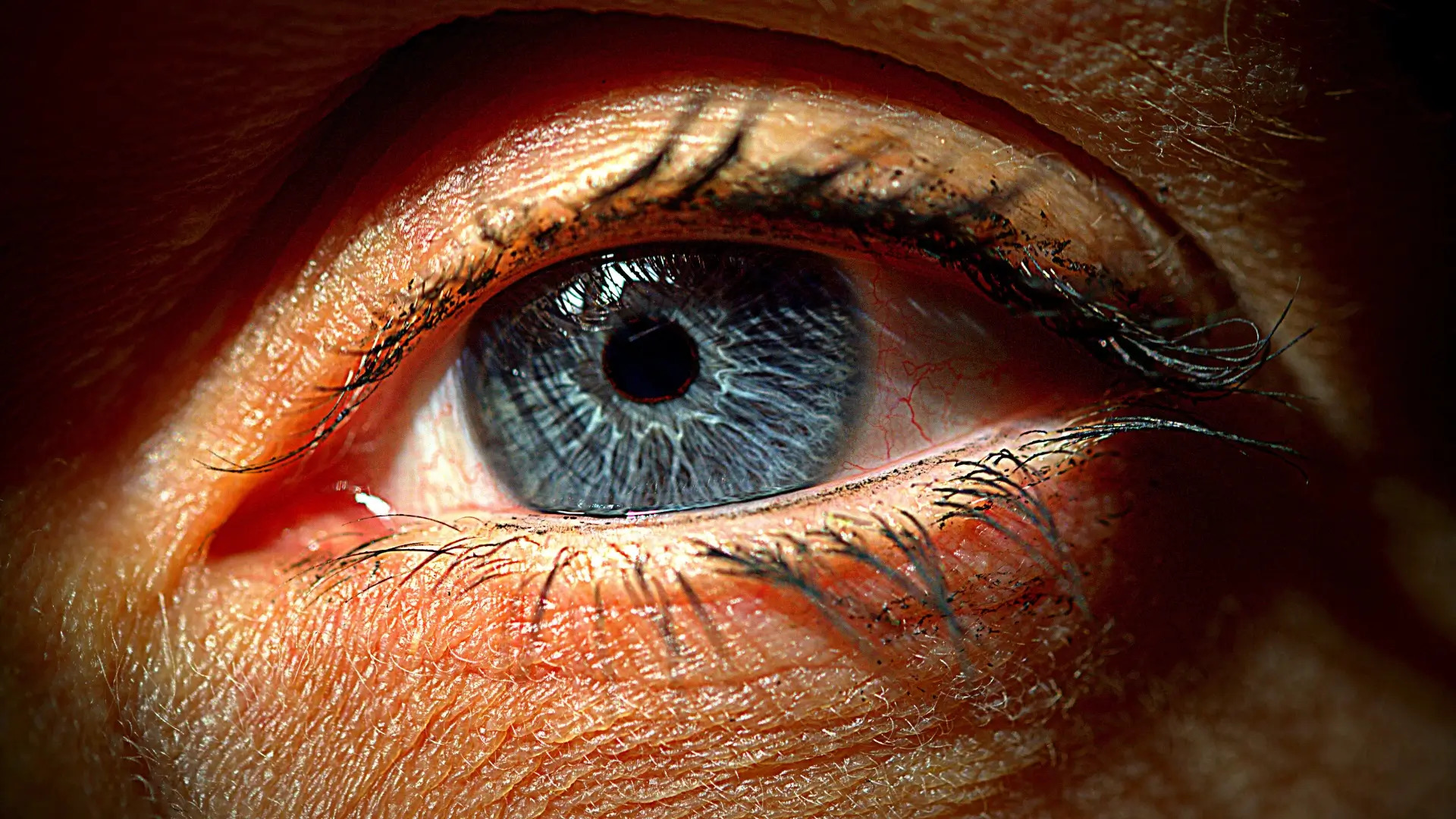
The bimatoprost in the Latisse solution may cause eye color changes. This occurs due to the increasing melanin in the iris. A study believed that if Latisse eye color change happens, this rare side effect is common among individuals with green or hazel eyes. Patients with light brown eyes may also notice this effect.
This study also highlights that the longer the medication is used, the darker the eyes could be. According to Ro, the increased iris color pigmentation typically begins around the pupil’s edge and spreads to the iris. As individuals may find this rare side effect concerning, it does not cause medical problems with the eyes.
However, this pigmentation may be permanent but does not cause dramatic eye changes. In addition to Latisse before and after photos, Latisse has proven its safety and efficacy in addressing hypotrichosis in several clinical studies.
A controlled trial demonstrated that daily administration of Latisse bimatoprost ophthalmic solution 0.03% over one year proved effective and well-tolerated in individuals suffering from idiopathic and chemotherapy-induced hypotrichosis.
Unfortunately, postmarketing surveillance does not reliably show the frequency of Latisse eye color change side effect, and no further studies have targeted this rare symptom. This requires medical professionals and patients to monitor one’s condition to manage this rare side effect.
Monitoring and Managing Eye Color Changes
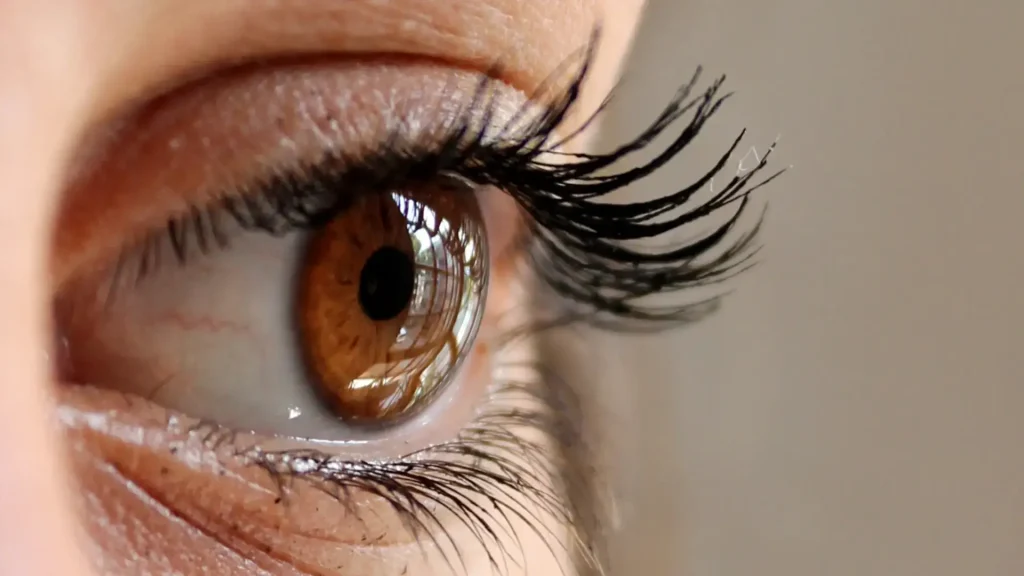
Patients using Latisse should be aware of the potential risk of eye color changes, a rare but possible side effect. Medical professionals must discuss this risk, along with other potential side effects, during consultations.
Patients should regularly inspect their eyes in a well-lit environment, using a mirror to detect any noticeable changes in color. Documenting these observations can help track any developments over time and assist in reporting concerns promptly.
Managing Eye Color Changes If They Occur
If patients notice any signs of eye color changes, they should consult their healthcare provider immediately. The medical professional may advise discontinuing Latisse to prevent further changes and exploring alternative treatments for eyelash growth, such as castor oil, argan oil, or aloe vera. These natural options enhance eyelash appearance without the risk of altering eye pigmentation.
Prompt consultation with a licensed healthcare provider is essential for managing this rare side effect. A professional evaluation can help determine the cause of the color change and provide guidance on appropriate next steps, ensuring patient safety and peace of mind.
Conclusion
Latisse is FDA-approved for eyelash growth, but patients should be aware of potential side effects, including changes in eye color. While studies suggest that this side effect does not lead to any medical conditions, it remains a concern for many users.
It’s essential for individuals considering Latisse to discuss its suitability, safety, and treatment protocol with their healthcare provider. Adhering to the prescribed usage and promptly consulting a professional if unusual eye changes occur are key steps in ensuring a safe and effective treatment experience.
FAQs
1. Does Latisse actually cause changes in eye color?
Yes, Latisse may cause changes in eye color as a rare side effect, particularly in individuals with green or hazel eyes. However, individuals should know that this side effect may happen rarely.
2. Are there any known risks associated with Latisse use?
Besides the potential for eye color changes, typical side effects may include redness and itching. It’s essential to consult a healthcare professional before starting Latisse treatment.
3. How can eye color changes caused by Latisse be monitored and managed?
It is recommended to inspect the eyes regularly for noticeable color changes and consult a medical professional if any changes occur. If eye color changes occur, discontinuing Latisse treatment and seeking medical advice are suggested.
References
- LATISSE® (bimatoprost ophthalmic solution) 0.03%. (n.d.). Latisse.com. Retrieved September 10, 2024, from https://www.latisse.com/results
- Vohnoutka, E. (2021, August 12). Can Latisse change your eye color? Ro.co. https://ro.co/latisse/latisse-eye-color-change/#is-latisse-eye-color-change-permanent