A recent consensus guidelines review analyzed 3,424 botulinum toxin type A injections for dystonia and spasticity, revealing significant dose variabilities of up to six times between individual muscles. This highlights the critical role of precise unit dosing algorithms in achieving optimal therapeutic outcomes and minimizing adverse events in clinical practice.
With this in mind, selecting the correct dosage is particularly crucial when using products like Innotox. Known for its highly purified formulation, Innotox requires tailored unit doses and specific dilution protocols to ensure consistent diffusion and clinical efficacy without compromising safety.
In this article, we will provide a comprehensive Innotox dosage chart, along with step-by-step administration instructions. We’ll also share practical preparation tips and safety checks to guide clinicians in delivering precise and effective treatments.
Key Takeaways
- Innotox offers a ready-to-use liquid formulation, eliminating the need for manual reconstitution and ensuring precise dosing and consistent outcomes for both practitioners and patients.
- The 4 U/0.1 mL concentration of Innotox enables predictable and symmetrical results, particularly in delicate areas like crow’s feet and glabellar lines, reducing the risk of dosing mistakes.
- Innotox has been shown to deliver visible wrinkle softening within 2–5 days, with full aesthetic effects typically achieved by Day 7. Results generally last 3–6 months, depending on the treatment area and dosage.
- While Innotox has not received FDA approval in the U.S., it has been cleared for aesthetic treatments by the Ministry of Food and Drug Safety (MFDS) in South Korea and is widely used in clinical applications in other regions.
- Innotox’s lack of albumin and animal-derived proteins may reduce the risk of allergic reactions and antibody formation, offering a safer option for patients with sensitivities.
- Clinicians transitioning from Botox to Innotox can use a 1:1 unit conversion, ensuring consistent dosing and similar treatment outcomes.
- Innotox’s long shelf life (up to 36 months) and room-temperature stability make it a more convenient option for clinics, compared to other botulinum toxin formulations that require strict cold storage.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Innotox, contact Medica Depot’s sales representatives and they will guide you on how to do so. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Innotox Dosage Chart by Indication and Treatment Area
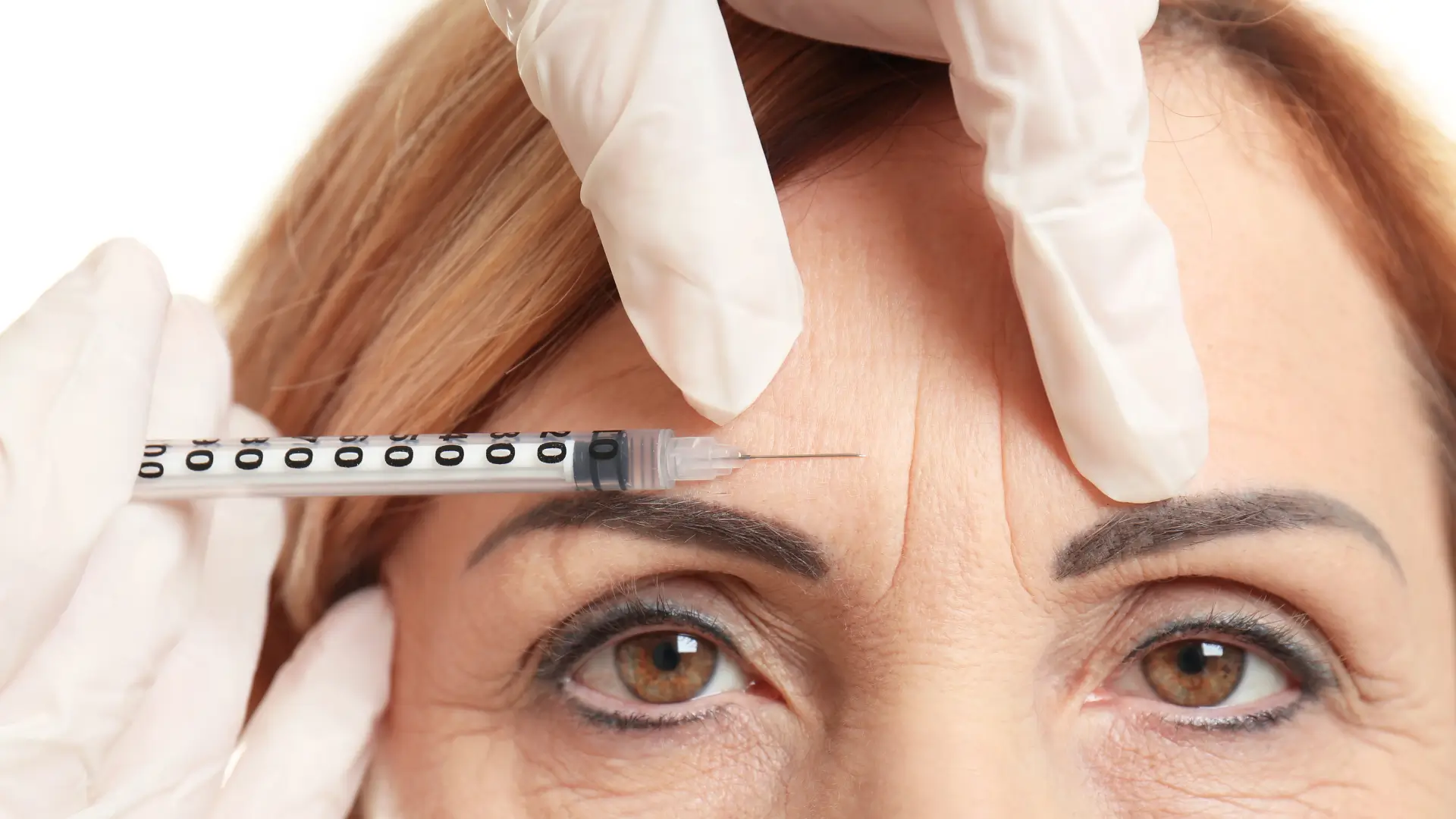
Medical professionals appreciate Innotox for its convenience and precise dosing. Its ready-to-use formulation allows practitioners to skip manual reconstitution, reducing appointment time and eliminating dilution errors. The unit ratio of Innotox is similar to Botox’s 1:1, making it easy for clinicians to apply familiar dosing protocols.
The pre-diluted liquid preparation of Innotox boosts dosing precision and ensures consistent outcomes across all treatment areas. While the manufacturer has not provided an official Innotox dosage chart, typical dosing guidelines for facial wrinkle smoothing include:
- Glabellar lines: 10–25 U (3–5 injection points)
- Forehead lines: 10–20 U (4–5 injection sites)
- Crow’s feet: 10–15 U per side
- Bunny lines: 5–10 U total
- Chin dimpling: 4–10 U
Despite its lack of FDA approval in the United States, Innotox has received clearance from the Ministry of Food and Drug Safety in South Korea for aesthetic treatments. However, some clinicians also use this botulinum toxin for clinical applications, such as:
- Axillary hyperhidrosis: 50 U per underarm
- Blepharospasm: 1.25–2.5 U per eye
Safe Injection Techniques When Administering Innotox
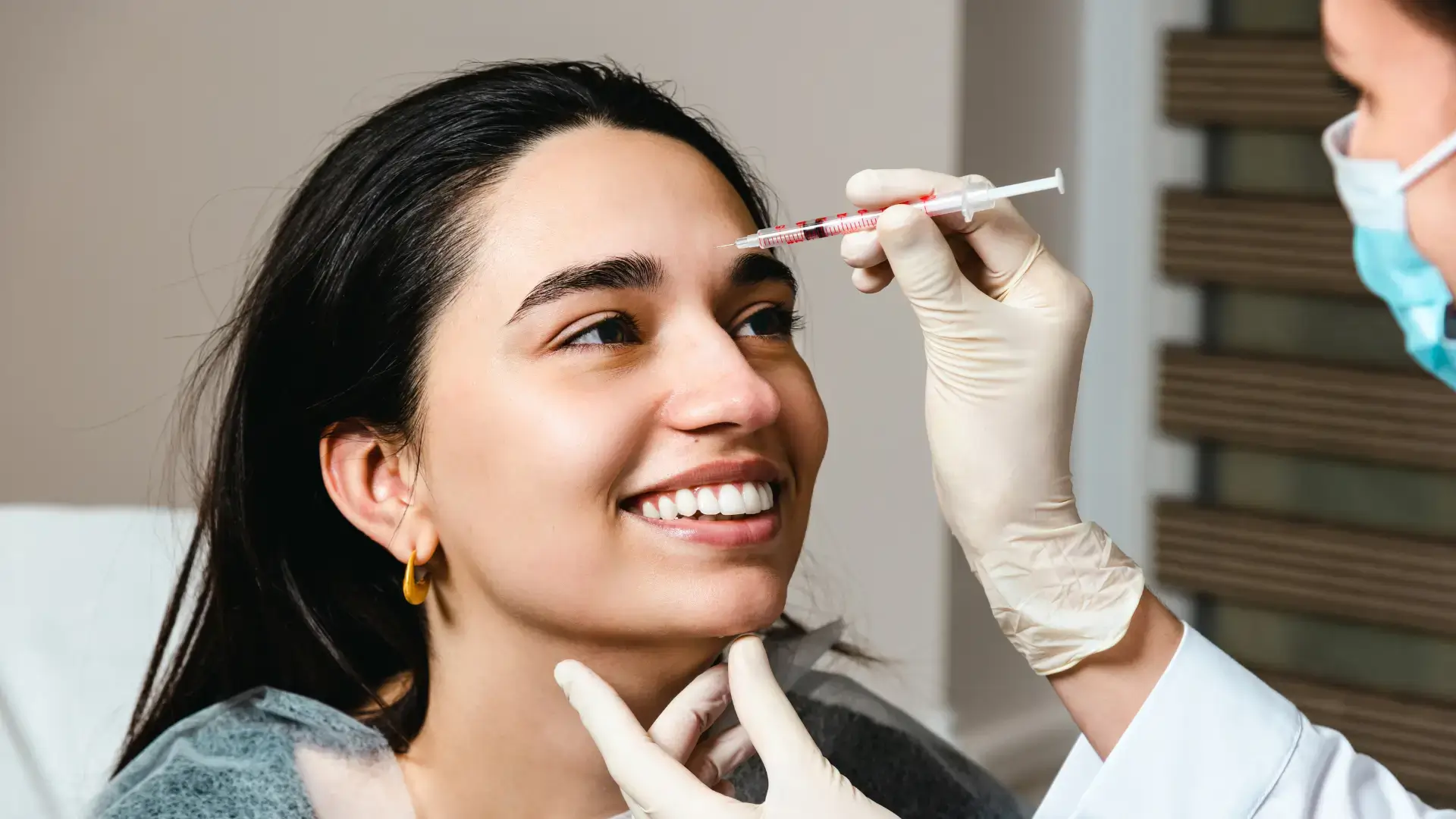
Ensuring precise administration and adhering to strict aseptic technique are key to maximizing safety, consistency, and patient confidence when using Innotox. Patients should always seek the expertise of licensed and trained medical professionals to avoid complications and achieve the best possible outcomes.
Practitioners should understand the proper administration of Innotox to ensure high patient satisfaction and safety. This begins with treatment preparation and post-treatment care, which are critical for a smooth and efficient Innotox procedure.
Pre-Injection Preparation
Before the injection process, medical practitioners must follow these steps to set the stage for a safe, error-free Innotox treatment:
- Review and evaluate the patient’s medical history, identify any contraindications, and set realistic expectations with the patient.
- Label every syringe with the patient’s name, date, and intended dose to ensure full traceability.
Injection Setup and Technique
Injectors must create a sterile working space and choose the correct tools to minimize the risk of contamination or infection. Consistent and anatomy-based injections ensure predictable diffusion and minimize common Innotox side effects like bruising.
- Map injection sites according to facial muscle activity and wrinkle pattern.
- For deep muscle relaxation, insert the needle at a 90° angle; for superficial zones, use a 45° angle.
- Slowly deposit 0.1 mL at each point, spacing injections 1–1.5 cm apart to avoid overlapping diffusion.
- After each injection, apply gentle pressure for a few seconds to reduce post-procedural bruising.
Post-Treatment Care and Follow-Up
Practitioners should guide patients through the first 24 hours to protect their results and reduce potential complications. Patients should be advised to avoid:
- Vigorous exercise, alcohol, and extreme temperature exposure for 24 hours.
- Touching or massaging treated areas during this period to prevent toxin migration.
Patients should sleep on their backs and apply a cold compress to the injection sites for the first 24 hours to soothe discomfort and limit bruising. Additionally, they should use broad-spectrum sunscreen each morning to protect delicate skin and support healing.
Follow-up visits should be scheduled within 2–4 weeks to assess efficacy, address side effects, and plan for future sessions. Practitioners should document accurate records and conduct timely check-ins to ensure optimal outcomes and address any reactions promptly.
Converting from Botox to Innotox: Dosing Considerations
When transitioning from Botox to Innotox, practitioners can use a straightforward 1:1 unit conversion, as both have matching clinical potencies. Both products contain Clostridium botulinum toxin type A (Hall strain).
Steps for Conversion and Administration
- Calculate the volume: 1 Innotox U = 0.025 mL (since 4 U/0.1 mL).
- Multiply the target units by 0.025 mL to determine the total injection volume.
- Use low dead-space insulin syringes (30–32 G) paired with sterile needles to deliver 4 U/0.1 mL per injection precisely.
- Keep the injection sites and spacing the same as the Botox routine, adjusting only the volume to match Innotox’s concentration.
- Onset of smoothing begins within 3–7 days, and a follow-up should be scheduled at two weeks to confirm the results.
Proper Storage and Handling of Innotox Vials
According to Medytox and MFDS, proper storage and handling of Innotox vials are essential to preserve both potency and sterility. To ensure impressive Innotox reviews, practitioners should store unopened vials in the original sealed packaging at 2°C–8°C. Under these conditions, vials remain stable and sterile for up to 36 months from the manufacturing date.
- Store vials upright in a dedicated pharmaceutical refrigerator and avoid exposure to food and direct light.
- Maintain the cold chain during transport and storage, ensuring temperature logs are continuously monitored.
- Discard any vial exposed to temperature excursions or past its expiration date to maintain quality.
Conclusion
Understanding the Innotox dosage chart and administration instructions is crucial for healthcare professionals aiming to achieve optimal patient outcomes. With its ready-to-use formulation, Innotox simplifies the injection process by eliminating the need for manual reconstitution, offering more precise dosing than traditional botulinum toxin products. Clinicians must follow proper preparation and injection techniques to maintain safety and efficacy throughout the treatment.
While Innotox lacks FDA approval in some regions, its widespread use in South Korea and clinical success highlight its potential as a reliable and effective treatment for wrinkle reduction. By adhering to recommended dosages and safety protocols, practitioners can meet patient expectations and deliver consistent, satisfying results.
FAQs
1. What is Innotox, and why is dosage important?
Innotox is a highly purified form of botulinum toxin type A used for aesthetic treatments. Precise dosage is crucial to ensure effective results and minimize the risks of adverse effects.
2. How does the dosing of Innotox compare to Botox?
Innotox can be converted using a 1:1 ratio with Botox due to their similar potencies, making it easier for practitioners to apply familiar dosing protocols.
3. What steps should practitioners take to ensure the safe administration of Innotox?
Practitioners should prepare a sterile environment, label syringes clearly, map injection sites according to muscle activity, and provide post-treatment care instructions to enhance safety and effectiveness.
References
Dressler D, Altavista MC, Altenmueller E, et al. Consensus guidelines for botulinum toxin therapy: general algorithms and dosing tables for dystonia and spasticity. Journal of Neural Transmission. 2021;128(3):321-335. doi:https://doi.org/10.1007/s00702-021-02312-4
Ministry of Food and Drug Safety (MFDS). Biological Products | (Biologics) Medytox. Mfds.go.kr. Published 2016. Accessed July 14, 2025. https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=70653