Did you know that prescriptions for tirzepatide (the active ingredient in Mounjaro) have multiplied in the United States? A recent analysis of 1.83 million adults with type 2 diabetes showed prescriptions rose from 4% to 12% of all glucose-lowering medications between January 2021 and December 2023. This rise highlights just how quickly Mounjaro is becoming part of everyday diabetes care.
As more patients begin using injectable treatments like Mounjaro, learning the proper way to use them is vital. Choosing the correct injection site, preparing the dose accurately, and handling the pen device with care not only improves effectiveness but also makes the experience more comfortable and reduces the risk of mistakes.
In this article, we’ll provide a clear, step-by-step guide on how to inject Mounjaro. From preparation and injection techniques to safe storage and aftercare, you’ll find practical tips designed to support safe, effective, and patient-centered use.
Key Takeaways
- Mounjaro (tirzepatide) is among the FDA-approved drugs or medications for type 2 diabetes. Its use in weight management is considered off-label in the U.S., while Zepbound (also tirzepatide) is approved for that purpose.
- Patients must not use Mounjaro injection if they have a history of hypersensitivity, medullary thyroid carcinoma, or MEN 2, as these increase the risk of serious complications.
- Mounjaro is delivered via a single-dose auto-injector pen designed for patient-friendly self-injection.
- Proper preparation includes washing your hands, inspecting the solution, confirming the expiration date, and gathering the necessary supplies (pen, alcohol swab, sharps container).
- Injections should be given once weekly, on the same day each week, at any time of day, with or without food.
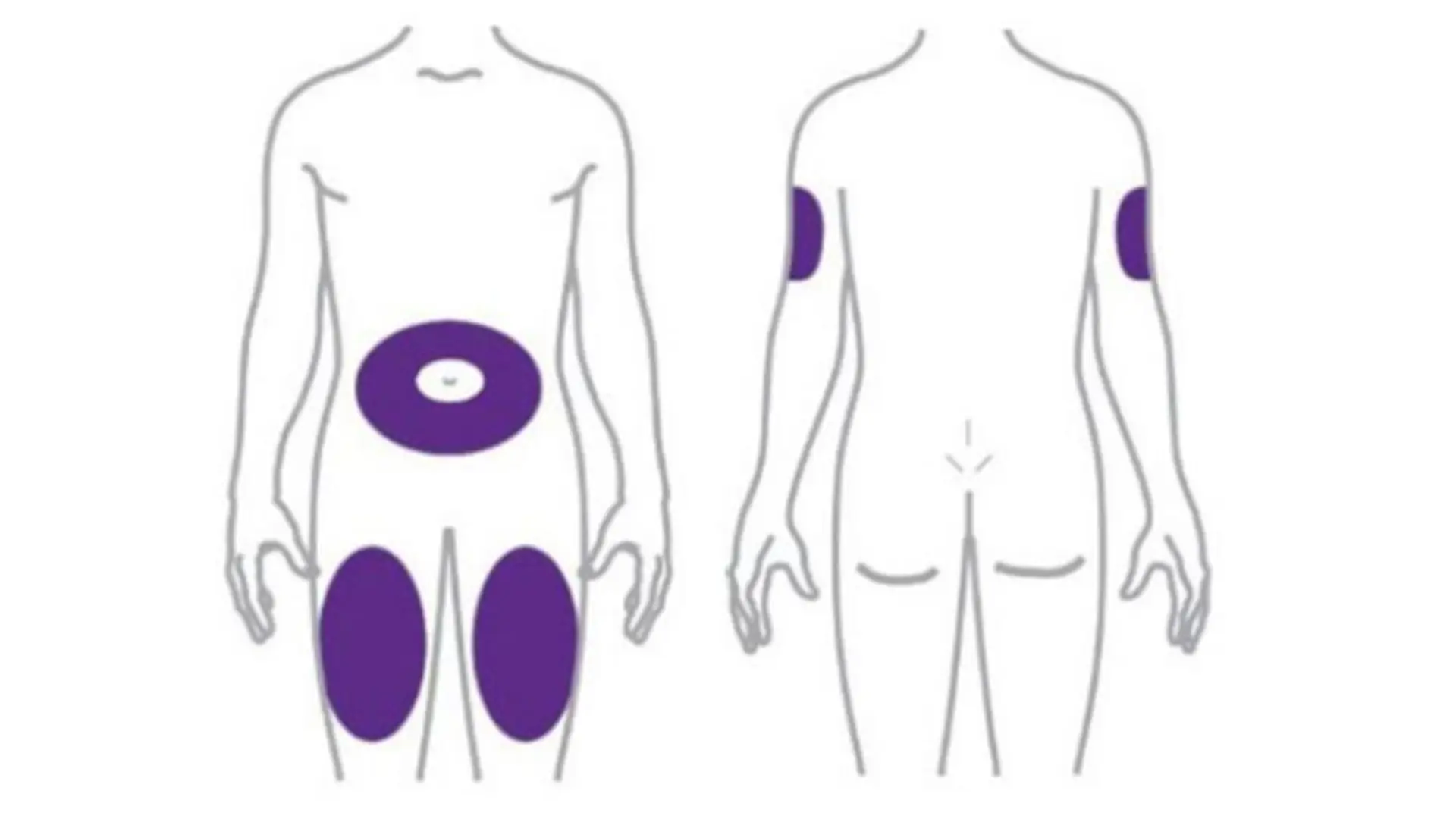
- Approved injection sites are the abdomen, thigh, or upper arm. Rotating sites helps minimize irritation and support consistent absorption.
- Safe disposal of the injection pen in an FDA-cleared sharps container immediately after use is necessary to prevent injuries.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Mounjaro, contact Medica Depot’s sales representatives and they will guide you on how to do so. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Preparing for a Mounjaro Injection: Steps and Supplies
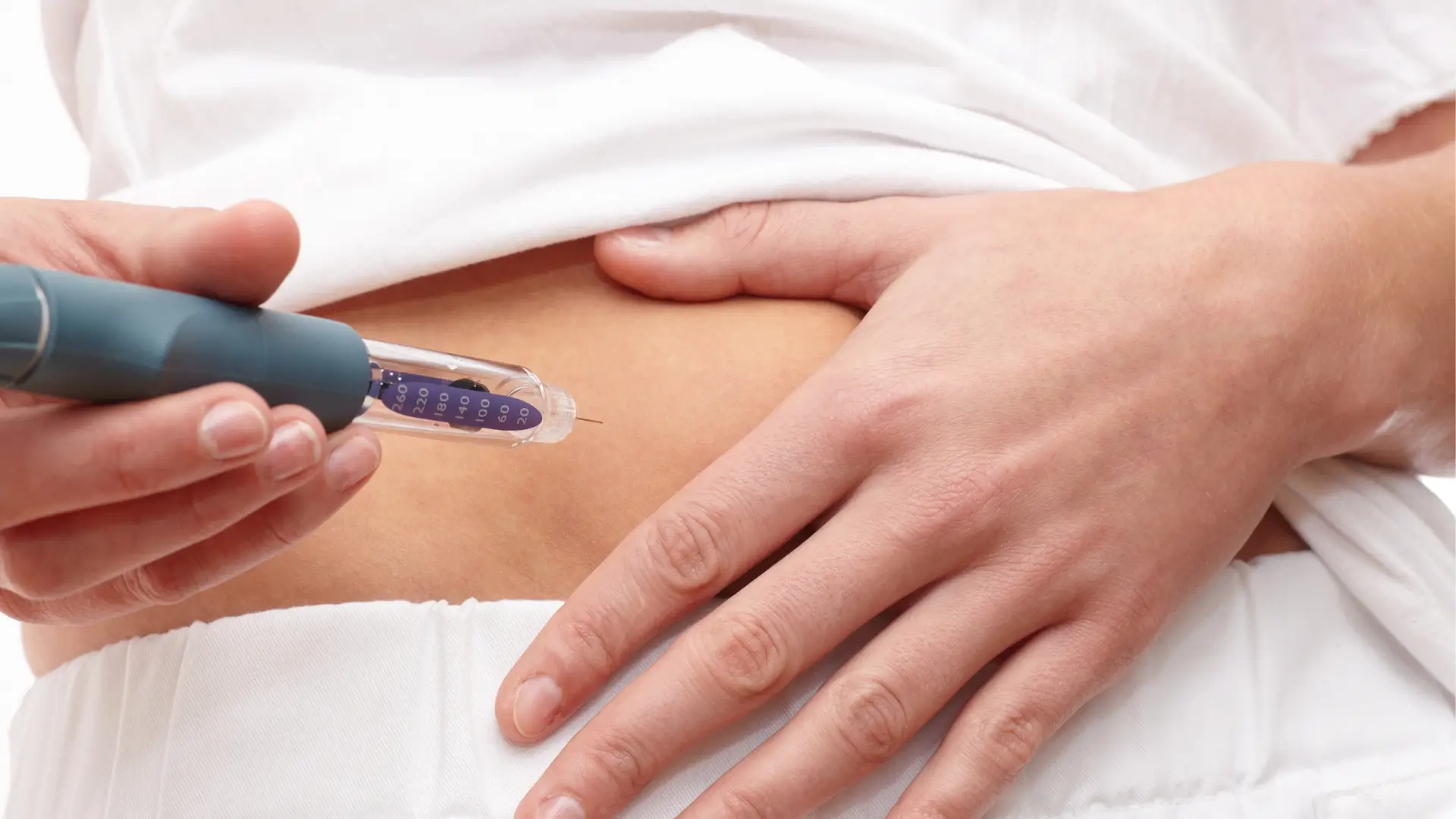
Before starting any new medication, healthcare professionals should carefully screen patients’ health histories to confirm suitability. For Mounjaro (tirzepatide), patients with type 2 diabetes must not have hypersensitivity to tirzepatide or any Mounjaro ingredients, nor a history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Excluding these conditions helps reduce the risk of serious side effects and complications.
It’s equally essential that practitioners receive proper training and guidance on how to inject Mounjaro pre-filled pen safely. According to the Mounjaro administration guide, this therapy uses a single-dose auto-injector pen designed for self injection, empowering patients to administer their medication with confidence.
Clinicians should teach patients how to prepare for their weekly Mounjaro injection:
- Follow the once-weekly schedule for best results. Begin with a 2.5 mg dose for the first four weeks, increasing by 2.5 mg every four weeks as directed.
- Wash hands thoroughly, check the Mounjaro pen’s name, dose, and expiration date, and inspect the solution—it should be clear, colorless to slightly yellow, and particle-free.
- Have all supplies ready: the single-dose Mounjaro pen, an alcohol swab, and an FDA-cleared sharps container.
How to Inject Mounjaro: Injection Sites and Technique for Administering Mounjaro
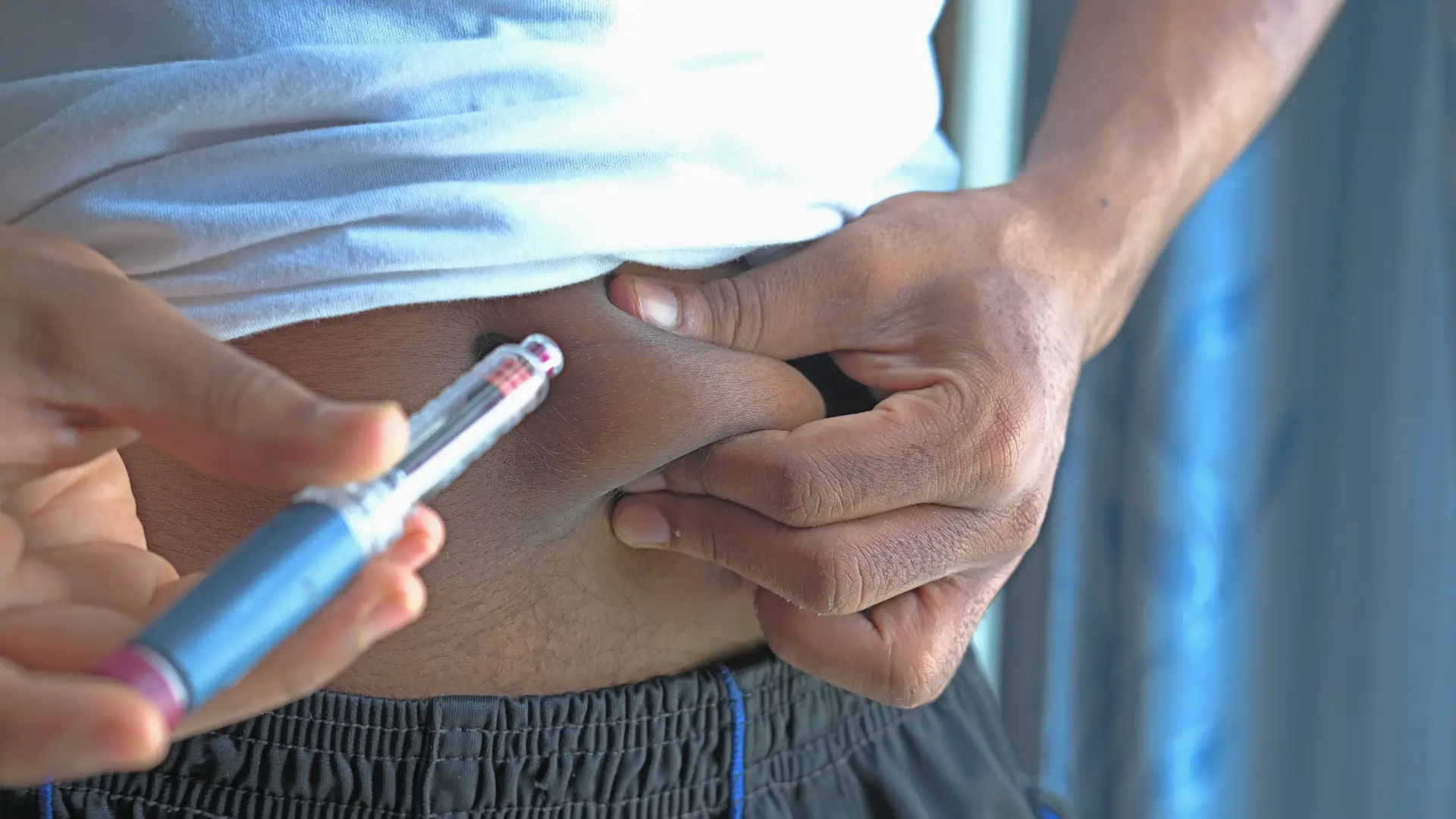
Proper storage and handling are essential. Mounjaro pens should be refrigerated at 2°C–8°C. Before injecting, patients can allow the pen to reach room temperature for comfort.
To inject correctly:
- Wash hands and clean the injection area.
- Pull off the gray base cap from the Mounjaro pen.
- Place the clear base flat against the skin at the chosen injection site.
- Unlock the pen’s safety cover and press the purple injection button.
- Hold the needle pen in place until it delivers the full dose (counter reads “0”) and you hear a click.
- Remove the injection pen from the skin and immediately dispose of the entire single-dose pen in an FDA-cleared sharps container.
Approved injection sites include the abdomen, thigh, or upper arm. For consistency and comfort, patients should inject once weekly, on the same day each week, at any time of day, with or without food.
Tips to Minimize Pain and Discomfort When Using Mounjaro Pens
To reduce discomfort, practitioners should advise patients to rotate the injection site. Switching between the abdomen, thigh, and upper arm helps maintain absorption and prevents irritation or tissue damage.
Providing patients with clear expectations can make Mounjaro injections less intimidating. Understanding the needle design, safety cover, and pre-filled pen function helps build confidence and reduce hesitation.
Some side effects are common, especially early in injection treatment. These may include:
- Nausea
- Diarrhea
- Decreased appetite
- Vomiting
- Constipation
- Dyspepsia (indigestion)
- Abdominal pain
Most symptoms resolve within a few days, but patients should always consult their healthcare provider if discomfort persists. Following the recommended preparation and administration steps often leads to a smoother experience and more effective Mounjaro injection results.
Patient Education for Self-Injecting Mounjaro at Home
Adults with type 2 diabetes or those exploring treatment for weight management should discuss their options with a qualified healthcare provider. It’s important to clarify that while Mounjaro is FDA-approved for type 2 diabetes, its use for weight management is considered off-label in the U.S. The related medication Zepbound (tirzepatide) carries FDA approval for chronic weight management.
Healthcare providers should be trained and certified to prescribe and teach proper injection techniques. Their expertise helps patients safely use Mounjaro as part of a larger care plan, which should include a balanced diet and regular physical activity.
Patients should always review the official Instructions for Use, Medication Guide, and prescribing information provided with their prescription. This reinforces safe practices and supports confidence with self-injection.
Conclusion
Correctly injecting Mounjaro is crucial for effective diabetes care and overall health outcomes. By understanding how to prepare the pen, choose the right injection site, and follow proper technique, patients can use the medication safely and confidently.
Rotating injection sites helps reduce discomfort, and monitoring for side effects ensures that any issues are addressed quickly. With the right guidance, patients can successfully self-administer Mounjaro and maintain better control of their diabetes, leading to improved quality of life.
FAQs
1. What are the recommended injection sites for Mounjaro?
The approved injection sites are the abdomen, thigh, and upper arm. Rotating among the approved areas of the Mounjaro injection helps minimize discomfort and skin irritation.
2. What should I check before injecting Mounjaro?
Make sure the Mounjaro pen is not expired and that the solution dose is clear and particle-free. Allow the pen to reach room temperature for added comfort.
3. How do I properly dispose of the Mounjaro injection pen?
Dispose of the entire used pen in an FDA-cleared sharps container immediately after injection to prevent needle stick injuries and ensure safe disposal.
References
Ostrominski JW, Ortega-Montiel J, Tesfaye H, et al. Trends in Utilization of Glucose- and Weight-Lowering Medications After Tirzepatide Approval in the United States. Annals of Internal Medicine. Published online April 15, 2025. doi:https://doi.org/10.7326/annals-24-02870
Eli Lilly and Company. Mounjaro Single-Dose Pen Instructions for Use. uspl.lilly.com. Accessed September 15, 2025. https://uspl.lilly.com/mounjaro/mounjaro.html#ug0