Did you know that 95% of patients with neovascular age-related macular degeneration (wet AMD) treated with Eylea (aflibercept) were able to maintain best-corrected visual acuity? They achieved this over the course of 52 weeks. Even more impressive, these patients experienced a loss of fewer than 15 letters. The results of this study make Eylea a non-inferior alternative to the commonly used monthly anti-VEGF therapies.
But Eylea isn’t just effective for wet AMD. It has shown remarkable results in treating other serious conditions like diabetic macular edema (DME) and retinal vein occlusion (RVO), consistently improving vision and reducing central retinal thickness. Thanks to its flexible dosing regimens, clinicians can strike the right balance between reducing treatment burden and maintaining long-term disease control.
In this article, we’ll explore Eylea’s clinical success, review key outcomes from clinical trials, and examine real-world evidence. We’ll also discuss important safety considerations, and provide practical insights on how to optimize patient care protocols for the best possible results.
Key Takeaways
- Eylea (aflibercept) has shown strong visual acuity gains in neovascular age-related macular degeneration (wet AMD), diabetic macular edema (DME), and macular edema following retinal vein occlusion (RVO). 95% of patients maintained best-corrected visual acuity (BCVA) over 52 weeks in clinical trials.
- Eylea delivers significant anatomical improvements. These include reductions in central retinal thickness (CRT), which helps preserve vision. It also supports sustained disease control in conditions like wet AMD, DME, and RVO.
- The treat-and-extend dosing regimen allows for extended dosing intervals. Eylea 2 mg shows significant visual gains and anatomical stability even with 8-week or longer intervals between injections in clinical studies.
- Real-world data from over 40,000 patients treated with Eylea HD (8 mg) show comparable vision improvements and fluid control to clinical trials. These results reinforce Eylea’s efficacy and flexibility in practice, even with longer dosing intervals.
- Patient characteristics, such as baseline visual acuity and early OCT response, play a significant role in predicting positive outcomes with Eylea. Adherence to the full loading phase is critical for achieving stable results and maximizing treatment benefits.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Eylea, contact Medica Depot’s sales representatives and they will guide you on how to do so. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Success in Visual Acuity Gains Across Indications

Intravitreal aflibercept treatments, especially Eylea (aflibercept), have shown impressive best-corrected visual acuity (BCVA) gains in clinical trials for various retinal conditions as an anti-VEGF therapy. Conditions include wet macular degeneration, diabetic macular edema (DME), and macular edema following retinal vein occlusion (RVO). Pivotal trials consistently show rapid visual improvements, sustained stability, and high rates of ≥15-letter gains, making Eylea a cornerstone medicine in treating these conditions.
According to Bayer Ophthalmology, here are the notable outcomes from clinical trials:
- nAMD: Treat-and-extend dosing with Eylea 2 mg resulted in patients receiving Eylea achieving sustained visual acuity gains up to week 16. The VIEW 1 and 2 clinical trials demonstrated mean BCVA gains of +7.6 letters with Q8W dosing and +7.9 letters with Q4W dosing, both comparable to ranibizumab.
- DME: In trials, Eylea showed superior visual acuity improvements, particularly in patients with a baseline BCVA of less than 69 letters. Moreover, patients treated with Eylea had a 30% higher chance of gaining ≥3 lines compared to those treated with another anti-VEGF medicine like ranibizumab in the first year.
- RVO: The COPERNICUS trial demonstrated that patients receiving Eylea 2 mg had an average gain of 20.2 letters, compared to -5.5 in the sham group after two months. 69% of these patients gained ≥3 lines from baseline. Eylea treatment also achieved superior vision gains with fewer injections, highlighting better treatment success compared to bevacizumab.
Anatomical Improvements and Fluid Reduction with Aflibercept
One of Eylea’s most notable benefits is its ability to dry the macula and thin the retina, producing significant anatomical improvements across conditions like nAMD, DME, and RVO. Clinical trials have consistently reported central retinal thickness (CRT) reductions, which play a key role in improving both visual acuity and anatomical structure.
By targeting VEGF, Eylea reduces retinal swelling, resolves fluid, and helps restore standard eye structure. This anatomical response is a major contributor to Eylea success in vision preservation.
In studies involving nAMD, patients who received Eylea experienced a noticeable reduction in CRT after the first injection. For example, the VIEW trial showed a reduction in CRT that was maintained through the second year of treatment.
For DME, the VIVID and VISTA trials demonstrated a CRT reduction of 192.4 microns and -183.1 microns for Eylea-treated patients by week 52. By week 100, these patients maintained significant CRT reductions of -195.8 microns and -191.1 microns.
In the COPERNICUS and GALILEO studies for RVO, Eylea HD resulted in a maximal visual acuity improvement by month 3. It then had a steady anatomical response, particularly in CRT, maintained through month 6. These improvements were statistically significant and lasted up to week 52, confirming the effectiveness of anti-VEGF therapy with Eylea (aflibercept).
Taken together, these findings emphasize that patients receiving Eylea injections experience not only visual gains but also strong anatomical outcomes. This dual benefit reinforces Eylea’s role as a reliable and effective medicine for retinal diseases, ensuring a high success rate across diverse patient groups.
Real-World Outcomes Compared to Clinical Trials
Real-world evidence reinforces the efficacy of Eylea, as clinical data consistently match what has been seen in controlled trials. Studies like VIEW, ALTAIR, VIVID, VISTA, PULSAR, GALILEO, and COPERNICUS demonstrate that patients treated with Eylea continue to experience visual acuity gains and anatomical improvements. This includes even outside the highly controlled environments of clinical trials.
At ARVO 2025, Regeneron shared data from nearly 40,000 Eylea HD-treated patients. The data, sourced from the IRIS Registry and the Vestrum Health Retina Databases, showed that real-world outcomes for Eylea HD (8 mg) were comparable to results from pivotal trials. Patients demonstrated vision improvements and fluid control, with clinicians safely extending dosing intervals while maintaining BCVA and OCT anatomy.
Importantly, this data highlighted how clinicians were able to adapt Eylea dosage by extending dosing intervals. They are based on individual patient responses, resulting in fewer injections without compromising efficacy.
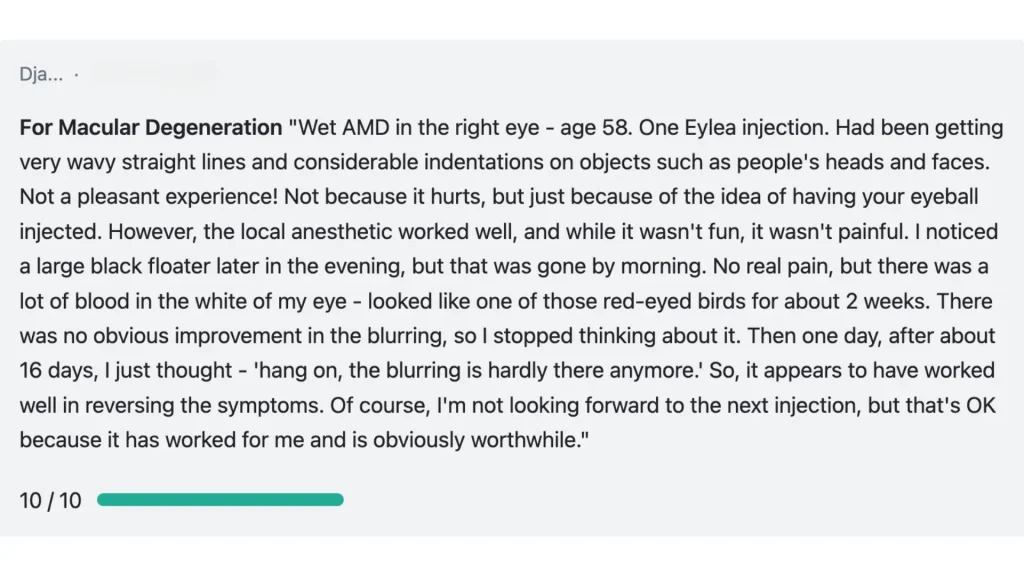
On Drugs.com, Eylea received an average rating of 7.1 out of 10 from patient reviews. Those using it for macular degeneration reported a 7.3 rating. These reviews generally reflect positive outcomes, with many patients sharing improvements in vision and disease control.
Predictors of Positive Response to Eylea Treatment
Identifying which patients are likely to respond well to Eylea can help optimize treatment plans. By assessing baseline vision, lesion characteristics, early OCT changes, and adherence to the loading phase, clinicians can personalize treatments to maximize visual gains and disease control.
Key predictors of a strong response include:
- Baseline Visual and Lesion Features: Patients with lower starting BCVA (e.g., <20/80) often experience larger letter gains. While their final acuity may still be lower than patients with better baseline vision, they still show significant improvements.
- Early OCT Response: If patients achieve fluid-free OCT after their first injection, it often indicates durable VEGF suppression and can support extending dosing intervals.
- Loading-Phase Adherence: Completing the full loading phase with three consecutive monthly 2 mg injections is critical for building stable drug levels. Delays or missed doses during the loading phase can lead to fluid return and smaller visual gains.
- Patient Characteristics: Patients under 75 years old, with fewer vascular comorbidities, tend to respond more strongly to treatment. Ensuring patients are in optimal systemic health can further enhance the effectiveness of aflibercept treatment.
Conclusion
Eylea (aflibercept) continues to demonstrate strong success rates across several retinal conditions. It works specially well in maintaining visual acuity for patients with neovascular age-related macular degeneration (wet AMD). Its effectiveness in clinical trials and real-world settings also underscores its broad utility in treating diabetic macular edema and retinal vein occlusion.
By carefully considering predictors of positive response and tailoring treatment based on individual patient needs, clinicians can help achieve the best possible outcomes. As a result, they improve the quality of life for patients dealing with serious eye conditions.
FAQs
1. What is the success rate of Eylea for maintaining visual acuity in patients with neovascular age-related macular degeneration?
Eylea maintains visual acuity in 95% of patients with neovascular age-related macular degeneration after one year of treatment.
2. How does Eylea perform in improving vision for other eye conditions?
Eylea shows significant visual acuity gains in diabetic macular edema and retinal vein occlusion. This makes it effective for these conditions as well.
3. What are the key factors that predict a positive response to Eylea treatment?
Key factors include baseline vision, characteristics of the lesion, early OCT changes, and adherence to the treatment plan.
References
Bayer Ophthalmology. EYLEA® 2mg in nAMD. Bayer.com. Accessed August 12, 2025. https://ophthalmology.bayer.com/eylea-2mg/indications/namd
Regeneron Pharmaceuticals, Inc. HIGHLIGHTS of PRESCRIBING INFORMATION | EYLEA® (Aflibercept). 2024. Accessed August 12, 2025. https://www.regeneron.com/downloads/eylea_fpi.pdf
Eylea, INN-Aflibercept | ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Bayer.com. Published 2022. Accessed August 12, 2025. https://www.bayer.com/sites/default/files/eylea-2mg-smpc-nov-2022.pdf