Actemra (tocilizumab) is an immunosuppressive infusion therapy commonly prescribed for rheumatoid arthritis, giant cell arteritis, and cytokine release syndrome. Its role in managing severe inflammation has expanded in recent years, especially during the COVID-19 pandemic. While clinically effective, Actemra affects the immune system, which may increase susceptibility to infections and other side effects.
Understanding the full spectrum of Actemra’s side effects is essential for ensuring patient safety, managing expectations, and supporting informed clinical decisions. Side effects can range from mild reactions like headaches and gastrointestinal discomfort to serious complications involving the liver, cardiovascular system, or immune response.
This article provides a comprehensive overview of Actemra’s side effects, offering patients and healthcare providers detailed insights into its safety profile and clinical considerations for safer therapy management.
Key Takeaways
- Actemra (tocilizumab) is an effective IL-6 receptor antagonist used to treat rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis, and other inflammatory conditions.
- While generally well-tolerated, common side effects include upper respiratory tract infections, headache, hypertension, and injection site reactions, typically resolving within days.
- Serious and rare side effects such as gastrointestinal perforations, hepatotoxicity, and serious infections require careful screening, especially in patients with pre-existing conditions or those receiving concurrent immunosuppressive therapies.
- Laboratory abnormalities associated with Actemra include elevated liver enzymes, reduced neutrophil counts, and increased lipid profiles, necessitating structured monitoring protocols for early detection.
- Proper administration and individualized dosing strategies are critical for minimizing side effects and ensuring patient safety during treatment.
- Adverse reactions should be managed through dose adjustments, temporary discontinuation, or supportive care, with serious events requiring immediate therapy suspension.
- Pharmacovigilance and adverse event reporting play an essential role in refining treatment protocols and ensuring ongoing safety assessments.
- Continuous patient education and proactive healthcare provider and patient communication are key to managing Actemra therapy effectively, supporting informed decision-making and optimizing outcomes.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. Buy Actemra online at Medica Depot today! Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Common Side Effects of Actemra

Actemra (tocilizumab) has demonstrated efficacy and safety as a prescription medicine for multiple conditions, including rheumatoid arthritis, systemic sclerosis with interstitial lung disease, giant cell arteritis, various forms of juvenile idiopathic arthritis, and COVID-19-related complications. Its versatility as an interleukin-6 (IL-6) receptor antagonist underscores its role in modern immunomodulatory medication therapy.
However, despite its favorable safety profile, it remains essential for healthcare providers to thoroughly discuss potential Actemra side effects with patients. Providing comprehensive information empowers patients to make informed treatment decisions and fosters realistic expectations regarding possible adverse reactions.
Mild side effects are more common and usually resolve without intervention. The most frequently reported side effects include:
- Upper respiratory tract infections
- Headache
- Hypertension
- Injection site reactions
According to prescribing data information, these reactions occur in at least 5% of patients. While generally transient, persistent or worsening symptoms warrant prompt medical evaluation with a doctor or in a clinic to prevent escalation into more significant health concerns. Education about these common effects improves treatment adherence and overall patient confidence in the medicine.
Serious and Rare Side Effects of Actemra
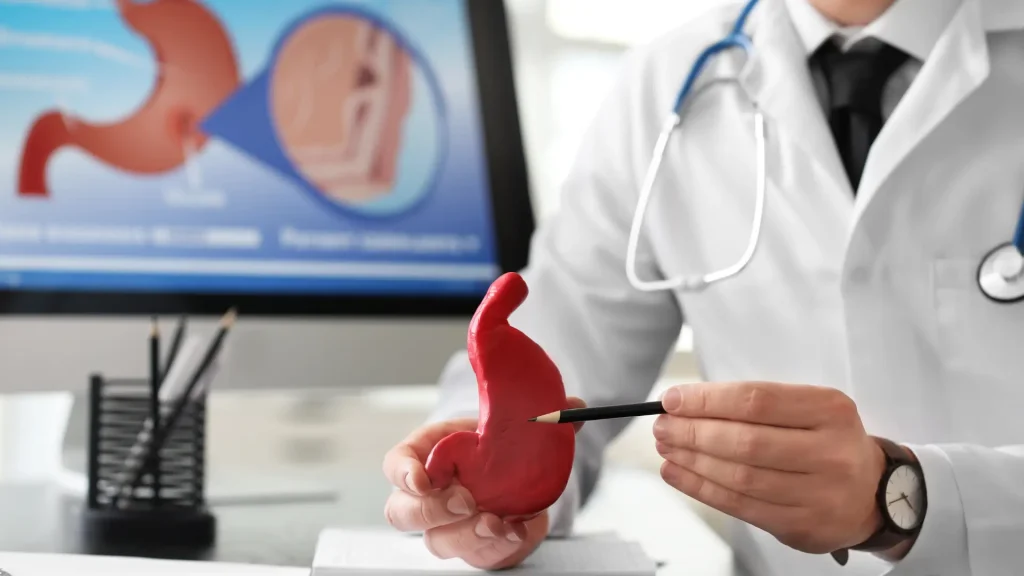
Beyond common side effects, serious adverse events associated with Actemra, though rare, must be carefully considered before initiating therapy. Factors such as improper Actemra administration, pre-existing conditions, or concurrent immunosuppressive therapies or medications can influence the likelihood of these complications.
These require immediate attention from a doctor or specialist. Notable serious side effects include:
- Gastrointestinal perforations, presenting with severe stomach pain and signs of peritonitis.
- Hepatotoxicity or liver problems, characterized by elevated liver enzymes and potential liver injury.
- Serious infection or allergic reaction, including tuberculosis, invasive fungal infections, and bacterial sepsis.
Patients with a history of intestinal lesions, existing liver problems, or concurrent use of other medicines face a greater risk. In these cases, thorough pre-treatment screening tests and ongoing monitoring are critical. A comprehensive risk–benefit analysis should guide clinical decisions, particularly for patients on corticosteroids, methotrexate, or similar agents.
By adhering to evidence-based administration protocols, clinicians can minimize serious adverse events while maintaining Actemra’s therapeutic efficacy.
Laboratory Abnormalities Associated with Actemra
Monitoring laboratory parameters is a crucial aspect of safe Actemra therapy. Regular tests and assessments help detect subclinical adverse effects, enabling early intervention before clinical symptoms develop. A doctor or provider must watch for these key laboratory abnormalities, which include:
- Liver Enzymes (ALT, AST): Elevations may occur, typically remaining asymptomatic but requiring observation.
- Neutrophil Counts (ANC): Actemra may lower ANC, necessitating baseline checks and periodic monitoring every 2–4 weeks.
- Lipid Profiles: Increases in LDL and HDL cholesterol are possible, requiring lipid panels at baseline, 4–8 weeks, and periodically thereafter.
Healthcare providers should establish structured monitoring schedules:
- Baseline assessments before therapy initiation.
- Liver function and neutrophil monitoring every 2–4 weeks.
- Lipid profile evaluations at specified intervals.
Should laboratory values exceed safety thresholds, clinicians must adjust treatment accordingly. This proactive approach ensures patient safety while preserving the therapeutic benefits of Actemra.
Adjustments to dosage or timing can reduce risks while maintaining effectiveness. These measures help clinics deliver safe care tailored to each patient’s needs.
Managing and Reporting Actemra Side Effects

Effective adverse effect management begins with individualized dosing strategies. Mild side effects, such as headache or dizziness, may only need careful monitoring. Moderate to severe effects, like allergic reaction or liver problems, could necessitate dose adjustments or temporary discontinuation. Severe complications require immediate cessation of therapy and further evaluation.
Healthcare providers must follow established protocols to mitigate risks. Key steps include:
- Adjusting treatment dosage based on patient tolerance and emerging side effects.
- Implementing supportive measures for manageable reactions.
- Discontinuing Actemra when serious adverse events are confirmed.
Additionally, robust pharmacovigilance is essential. Prompt reporting of adverse events to regulatory agencies not only enhances patient safety but also contributes to post-marketing surveillance data. Using standardized systems ensures consistent tracking, aiding in refining treatment guidelines and improving clinical outcomes.
Transparent communication with authorities, coupled with patient education, strengthens the overall safety framework of Actemra administration.
Conclusion
A detailed understanding of Actemra’s side effect profile is vital for both patients and healthcare providers. While most individuals tolerate the therapy well, vigilance regarding common adverse effects, such as infections or liver function alterations, is essential for safe, effective treatment.
Continuous monitoring, proactive management, and open dialogue between patients and clinicians foster a collaborative approach to therapy. This empowers patients to report side effects early, supports informed decision-making, and ultimately enhances overall treatment success.
By maintaining high standards of patient education and clinical oversight, healthcare providers can ensure that Actemra remains a valuable and safe option within the landscape of immunosuppressive therapies.
FAQs
1. What are the common side effects of Actemra (tocilizumab)?
Common side effects include infections, injection site reactions, and changes in liver function. Patients should be aware of these potential issues and monitor their health closely.
2. Why is it important to monitor side effects when taking Actemra?
Monitoring side effects is crucial because it can help enhance patient safety and improve treatment outcomes. Early detection of side effects allows for timely intervention and management.
3. How can patients effectively manage their therapy with Actemra?
Patients can manage their therapy effectively through ongoing education and open communication with their healthcare teams. Discussing concerns and reporting side effects promptly empowers patients to take an active role in their health management.
References
- Holtedahl R, Brox JI, Tjomsland O. Placebo effects in trials evaluating 12 selected minimally invasive interventions: a systematic review and meta-analysis. BMJ Open. 2015;5(1):e007331-e007331. doi:https://doi.org/10.1136/bmjopen-2014-007331
- Instructions for Use ACTEMRA® (AC-TEM-RA) (tocilizumab). Gene. Accessed May 12, 2025. https://www.gene.com/download/pdf/actemra_ifu.pdf