Manufacturer: Eli Lilly and Company Limited
Active Substance(s): TIRZEPATIDE
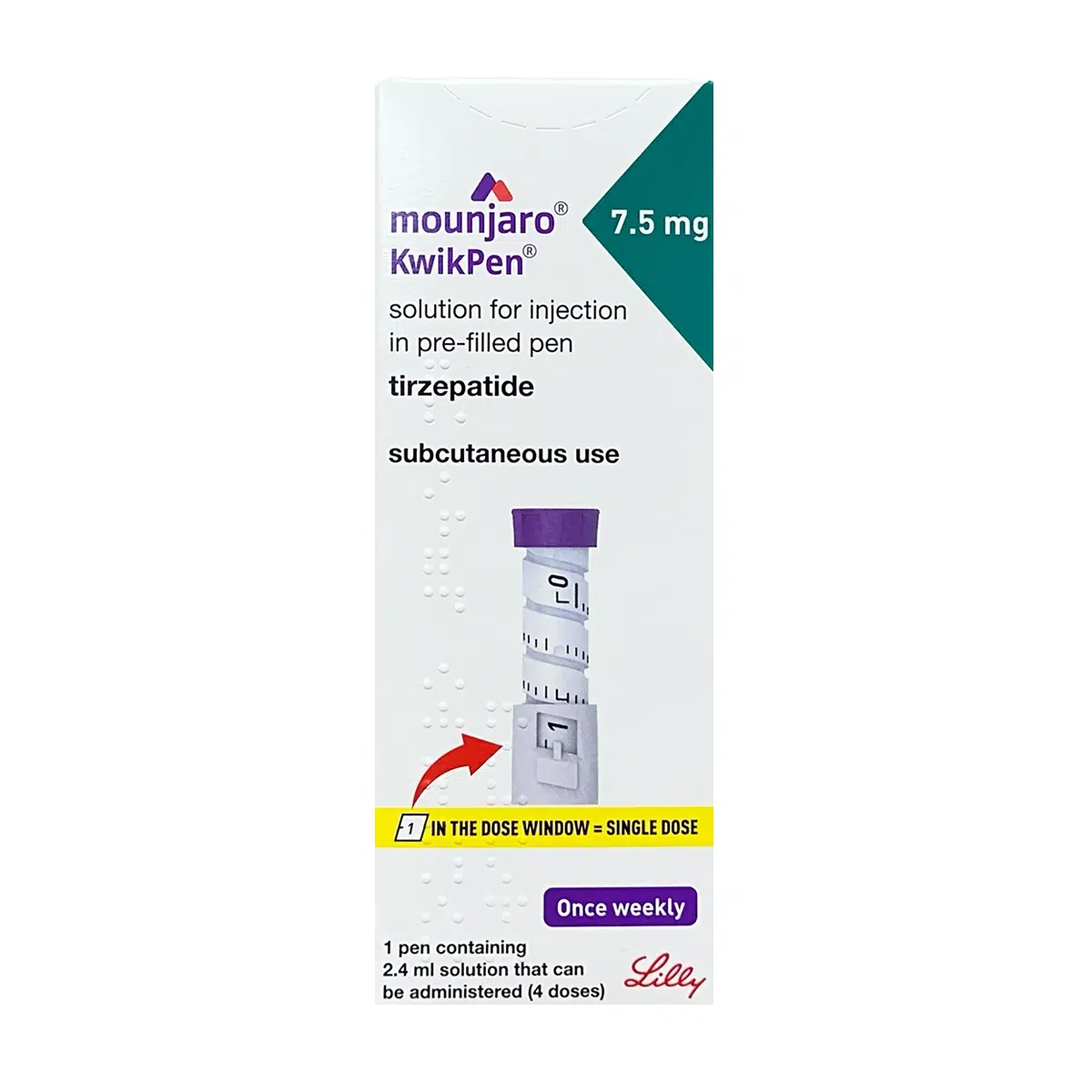
Strength: 7.5mg/0.6ml injection
Pack Size: 1 box with 4 pre-filled KwikPens
INFORMATION
What is MOUNJARO® 7.5mg KwikPen®
MOUNJARO® 7.5mg KwikPen® is a multi-dose, pre-filled injection device delivering 7.5 mg tirzepatide in 0.6 mL once weekly via subcutaneous injection. It is used for the treatment of adults with type 2 diabetes mellitus and as an adjunct for weight management in patients who meet clinical BMI thresholds. Each KwikPen® contains 2.4 mL of solution (total of 30 mg tirzepatide) and supplies four fixed doses. The solution is a clear, colourless to slightly yellow liquid, administered in the abdomen, thigh, or upper arm.
-
Dose per injection: 7.5 mg tirzepatide in 0.6 mL
-
Total pen content: 30 mg/2.4 mL (12.5 mg/mL)
-
Number of doses per pen: 4
-
Injection sites: Abdomen, thigh, upper arm
-
Administration: Subcutaneous only
What is the Administration Protocol for MOUNJARO® 7.5mg KwikPen®
The 7.5 mg dose is commonly used as an intermediate titration step in the dosing schedule. Administration occurs once weekly, with careful timing and site rotation to optimize tolerability.
-
Initiation: Start with 2.5 mg weekly for 4 weeks, then increase in 2.5 mg increments
-
Titration: 7.5 mg is a transitional dose before advancing to higher levels if tolerated
-
Maintenance doses: 5 mg, 10 mg, or 15 mg once weekly depending on patient needs
-
Missed dose protocol:
-
If ≤4 days late: inject as soon as possible
-
If >4 days late: skip and resume on next scheduled day
-
-
Injection technique:
-
Use a new sterile needle for each injection
-
Dial to 1 in the dose window (=0.6 mL)
-
Inject subcutaneously, hold for 5 seconds until dose window shows 0
-
-
Rotate injection sites to minimize local irritation
What are the Indications for MOUNJARO® 7.5mg KwikPen®
This dose is indicated as part of the broader therapeutic use of tirzepatide for metabolic disorders. It serves both in glycaemic regulation and in weight management for qualifying adults.
-
Indications:
-
Treatment of adults with type 2 diabetes mellitus, as monotherapy or with other antidiabetic medicines
-
Weight management in adults with BMI ≥30 kg/m²
-
Adults with BMI ≥27 to <30 kg/m² plus at least one weight-related comorbidity (e.g., hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, or prediabetes)
-
-
Contraindications:
-
Hypersensitivity to tirzepatide or excipients
-
-
Precautions:
-
History of pancreatitis
-
Severe gastrointestinal disease (e.g., gastroparesis)
-
-
Adverse effects:
-
Common: Nausea, diarrhoea, vomiting, abdominal pain, constipation
-
Hypoglycaemia risk increased with insulin or sulphonylureas
-
Mild to moderate injection-site reactions
-
Occasional gallbladder events
-
Rare: Acute pancreatitis, hypersensitivity
-
What is the composition of MOUNJARO® 7.5mg KwikPen®
The pen is formulated with tirzepatide as the active pharmaceutical ingredient, supported by excipients for stability and preservation.
-
Active substance: Tirzepatide 7.5 mg/0.6 mL
-
Total content per pen: 30 mg in 2.4 mL
-
Concentration: 12.5 mg/mL
-
Excipients: Disodium hydrogen phosphate heptahydrate, benzyl alcohol (~5.4 mg per 0.6 mL dose), glycerol, phenol, sodium chloride, sodium hydroxide, hydrochloric acid, water for injections
-
Sodium content: <1 mmol sodium per dose (sodium-free)
-
Appearance: Clear, colourless to slightly yellow solution
What Comes in the MOUNJARO® 7.5mg KwikPen® Package
The packaging ensures correct dosing support, though pen needles must be acquired separately.
-
Presentation: Pre-filled KwikPen® with 2.4 mL (4 doses)
-
Pack sizes: 1 or 3 pens per carton (availability may vary)
-
Needles: Not included; must be obtained separately
-
Documentation: Patient Information Leaflet (PIL) and Instructions for Use (IFU) supplied
Does MOUNJARO® 7.5mg KwikPen® cause side effects?
MOUNJARO® may produce side effects, primarily gastrointestinal in origin, which are dose-dependent and most frequent during early treatment phases.
-
Very common: Nausea, diarrhoea, vomiting, abdominal pain, constipation
-
Hypoglycaemia: Higher risk with concomitant insulin or sulphonylurea use
-
Injection-site reactions: Reported in up to 6% of patients, mild to moderate in severity
-
Gallbladder events: Reported in 1–2% of cases
-
Uncommon but serious: Acute pancreatitis, hypersensitivity reactions (e.g., angioedema, anaphylaxis)
-
Laboratory findings: Mild elevations in amylase and lipase
How should MOUNJARO® 7.5mg KwikPen® be stored?
Storage protocols are essential to ensure sterility, stability, and potency.
-
Unopened pens: Refrigerate at 2–8 °C
-
Do not freeze; discard if frozen
-
After first use: May store at ≤30 °C for up to 30 days
-
Discard after 30 days, regardless of remaining solution
-
Needles: Remove after each injection; discard in a sharps container
-
Keep out of reach of children
What is MOUNJARO® 7.5mg KwikPen®
MOUNJARO® 7.5mg KwikPen® is a multi-dose, pre-filled injection device delivering 7.5 mg tirzepatide in 0.6 mL once weekly via subcutaneous injection. It is used for the treatment of adults with type 2 diabetes mellitus and as an adjunct for weight management in patients who meet clinical BMI thresholds. Each KwikPen® contains 2.4 mL of solution (total of 30 mg tirzepatide) and supplies four fixed doses. The solution is a clear, colourless to slightly yellow liquid, administered in the abdomen, thigh, or upper arm.
-
Dose per injection: 7.5 mg tirzepatide in 0.6 mL
-
Total pen content: 30 mg/2.4 mL (12.5 mg/mL)
-
Number of doses per pen: 4
-
Injection sites: Abdomen, thigh, upper arm
-
Administration: Subcutaneous only
What is the Administration Protocol for MOUNJARO® 7.5mg KwikPen®
The 7.5 mg dose is commonly used as an intermediate titration step in the dosing schedule. Administration occurs once weekly, with careful timing and site rotation to optimize tolerability.
-
Initiation: Start with 2.5 mg weekly for 4 weeks, then increase in 2.5 mg increments
-
Titration: 7.5 mg is a transitional dose before advancing to higher levels if tolerated
-
Maintenance doses: 5 mg, 10 mg, or 15 mg once weekly depending on patient needs
-
Missed dose protocol:
-
If ≤4 days late: inject as soon as possible
-
If >4 days late: skip and resume on next scheduled day
-
-
Injection technique:
-
Use a new sterile needle for each injection
-
Dial to 1 in the dose window (=0.6 mL)
-
Inject subcutaneously, hold for 5 seconds until dose window shows 0
-
-
Rotate injection sites to minimize local irritation
What are the Indications for MOUNJARO® 7.5mg KwikPen®
This dose is indicated as part of the broader therapeutic use of tirzepatide for metabolic disorders. It serves both in glycaemic regulation and in weight management for qualifying adults.
-
Indications:
-
Treatment of adults with type 2 diabetes mellitus, as monotherapy or with other antidiabetic medicines
-
Weight management in adults with BMI ≥30 kg/m²
-
Adults with BMI ≥27 to <30 kg/m² plus at least one weight-related comorbidity (e.g., hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, or prediabetes)
-
-
Contraindications:
-
Hypersensitivity to tirzepatide or excipients
-
-
Precautions:
-
History of pancreatitis
-
Severe gastrointestinal disease (e.g., gastroparesis)
-
-
Adverse effects:
-
Common: Nausea, diarrhoea, vomiting, abdominal pain, constipation
-
Hypoglycaemia risk increased with insulin or sulphonylureas
-
Mild to moderate injection-site reactions
-
Occasional gallbladder events
-
Rare: Acute pancreatitis, hypersensitivity
-
What is the composition of MOUNJARO® 7.5mg KwikPen®
The pen is formulated with tirzepatide as the active pharmaceutical ingredient, supported by excipients for stability and preservation.
-
Active substance: Tirzepatide 7.5 mg/0.6 mL
-
Total content per pen: 30 mg in 2.4 mL
-
Concentration: 12.5 mg/mL
-
Excipients: Disodium hydrogen phosphate heptahydrate, benzyl alcohol (~5.4 mg per 0.6 mL dose), glycerol, phenol, sodium chloride, sodium hydroxide, hydrochloric acid, water for injections
-
Sodium content: <1 mmol sodium per dose (sodium-free)
-
Appearance: Clear, colourless to slightly yellow solution
What Comes in the MOUNJARO® 7.5mg KwikPen® Package
The packaging ensures correct dosing support, though pen needles must be acquired separately.
-
Presentation: Pre-filled KwikPen® with 2.4 mL (4 doses)
-
Pack sizes: 1 or 3 pens per carton (availability may vary)
-
Needles: Not included; must be obtained separately
-
Documentation: Patient Information Leaflet (PIL) and Instructions for Use (IFU) supplied
Does MOUNJARO® 7.5mg KwikPen® cause side effects?
MOUNJARO® may produce side effects, primarily gastrointestinal in origin, which are dose-dependent and most frequent during early treatment phases.
-
Very common: Nausea, diarrhoea, vomiting, abdominal pain, constipation
-
Hypoglycaemia: Higher risk with concomitant insulin or sulphonylurea use
-
Injection-site reactions: Reported in up to 6% of patients, mild to moderate in severity
-
Gallbladder events: Reported in 1–2% of cases
-
Uncommon but serious: Acute pancreatitis, hypersensitivity reactions (e.g., angioedema, anaphylaxis)
-
Laboratory findings: Mild elevations in amylase and lipase
How should MOUNJARO® 7.5mg KwikPen® be stored?
Storage protocols are essential to ensure sterility, stability, and potency.
-
Unopened pens: Refrigerate at 2–8 °C
-
Do not freeze; discard if frozen
-
After first use: May store at ≤30 °C for up to 30 days
-
Discard after 30 days, regardless of remaining solution
-
Needles: Remove after each injection; discard in a sharps container
-
Keep out of reach of children