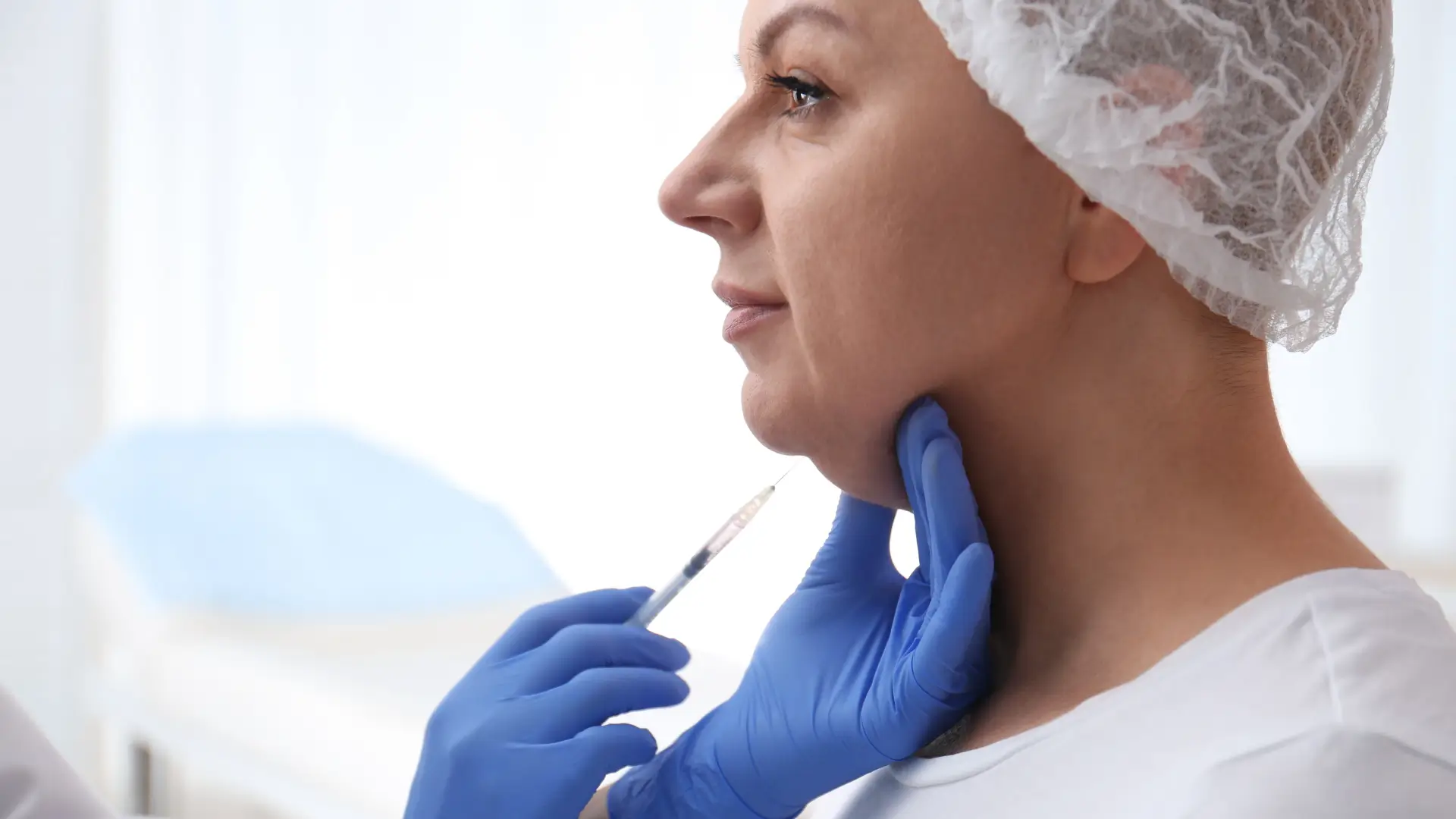
Aqualyx vs Kybella – Comparing Fat Dissolving Treatments
A landmark study revealed an interesting fact: while we experience weight loss, our bodies maintain a stable number of adipocytes (fat cells) throughout life, with only about 10% being renewed annually.
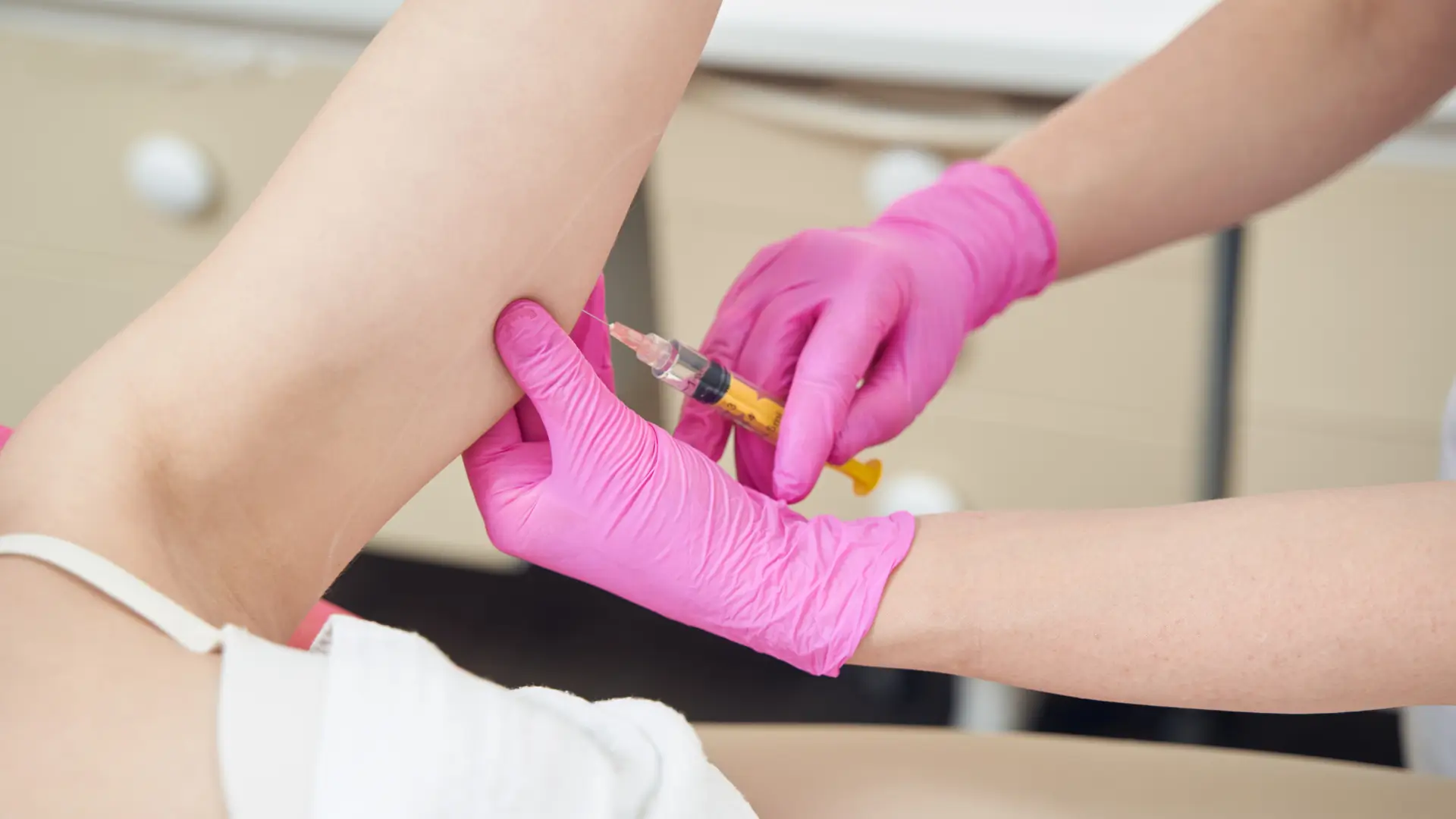
Aqualyx Fat Dissolving Injections – How Do They Work?
In 2023, U.S.
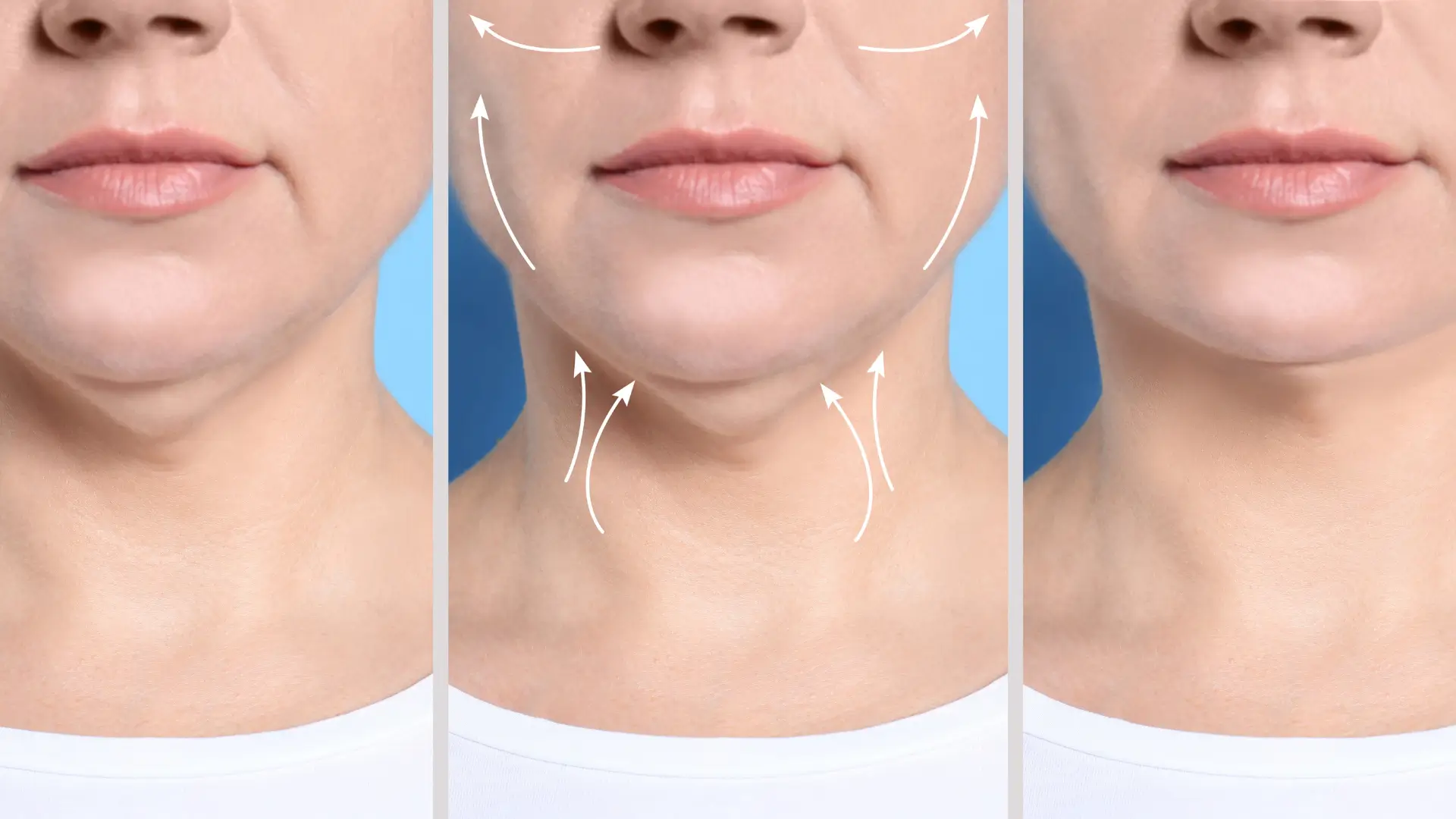
Aqualyx Before And After – With a Photo Gallery
Did you know that before-and-after photos are often the most powerful marketing tool for aesthetic clinics?
Eylea Success Rate – How Well Does It Work?
Did you know that 95% of patients with neovascular age-related macular degeneration (wet AMD) treated with Eylea (aflibercept) were able to maintain best-corrected visual acuity?
Eylea Dosage and Injection Frequency
Medication errors are a serious concern in healthcare, as incorrect dosing can lead to adverse reactions, treatment failures, and avoidable harm to patients.
Eylea Generic Name – About Aflibercept
Did you know that aflibercept, also known by its brand name Eylea, is approved for treating nine different clinical conditions?

Eylea for Macular Degeneration – Is It Successful?
Age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, especially those over 50.

Eylea Manufacturer – A Joint Venture by Regeneron and Bayer
As age-related vision loss continues to rise, with conditions like cataracts affecting over 24 million Americans and projected to rise to nearly 40 million by 2030, the need for reliable treatments is more pressing than ever.

Eylea vs Avastin – Wet AMD Treatments Compared
Wet age-related macular degeneration (wet AMD) is a leading cause of vision loss in older adults, responsible for around 90% of AMD-related blindness.

Eylea Prescribing Information
A product’s FDA-approved prescribing information is an essential resource for doctors and healthcare providers, offering critical details on how to use prescription medicines safely and effectively.
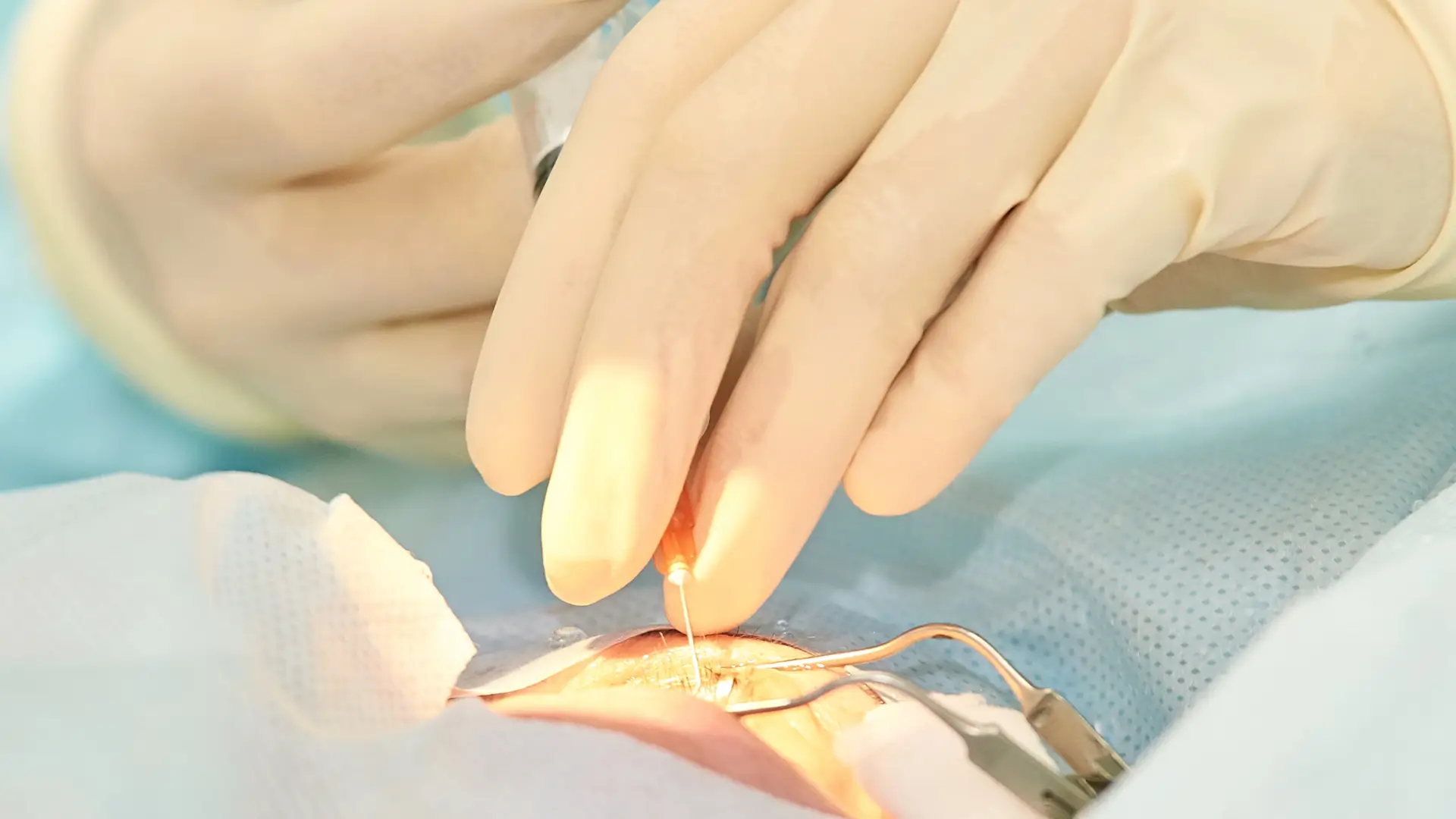
Vabysmo vs Eylea – Differences and Similarities
Did you know that nearly 6 million intravitreal injections (IVIs) were performed in just 2016?

How Long Does Eylea Stay In Your System?
Eylea (aflibercept) has become a cornerstone treatment for a variety of retinal conditions, including wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion.