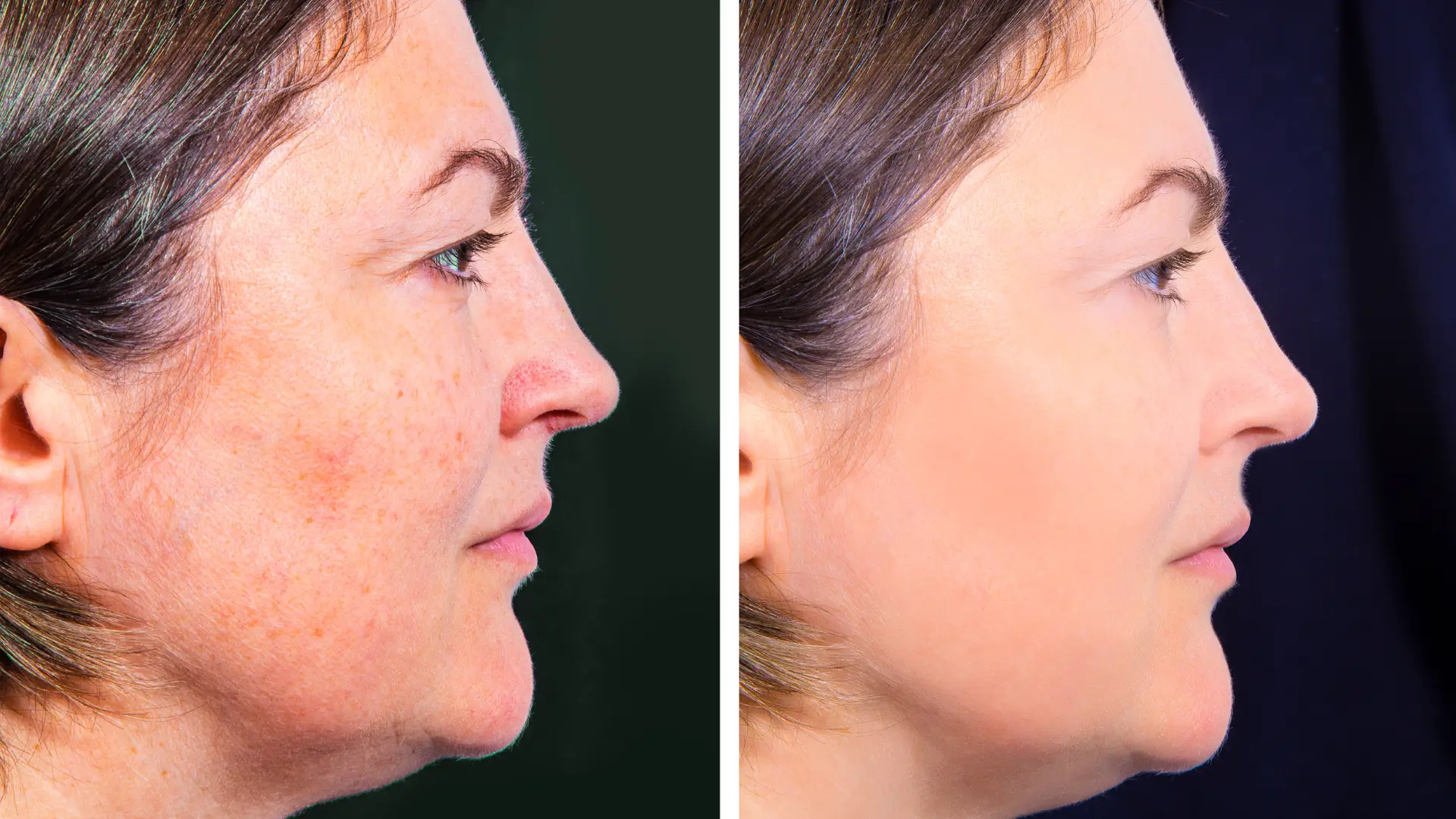
Lanluma Before and After – Bum, Face, Neck Pictures
A recent systematic review on the efficacy of poly‑L‑lactic acid in facial aesthetics reported an overall treatment success rate of 78%, underscoring its significant value in non-surgical rejuvenation.

PhilArt Before and After Discussion With a Photo Gallery
A recent review published in the International Journal of Molecular Sciences highlights that polynucleotide-based injections can improve skin elasticity and hydration by up to 30%, significantly enhancing overall dermal health.
PhilArt vs Profhilo – A Thorough Comparison
Minimally invasive cosmetic procedures have surged in popularity across the United States, with a 7% year-over-year growth in minimally invasive treatments, according to the 2023 American Society of Plastic Surgeons (ASPS) Statistics Report.
PhilArt vs Plinest – Pros & Cons of Polynucleotides
Polynucleotide injections have rapidly gained popularity in the field of aesthetic medicine, offering patients a non-surgical alternative for improving skin hydration, elasticity, and overall dermal health.
PhilArt Products – List and Indications
Minimally invasive cosmetic procedures continue to dominate the aesthetic industry.

Actemra Administration – Information for Practitioners
Proper medication administration is critical to ensuring patient safety and maximizing treatment efficacy.
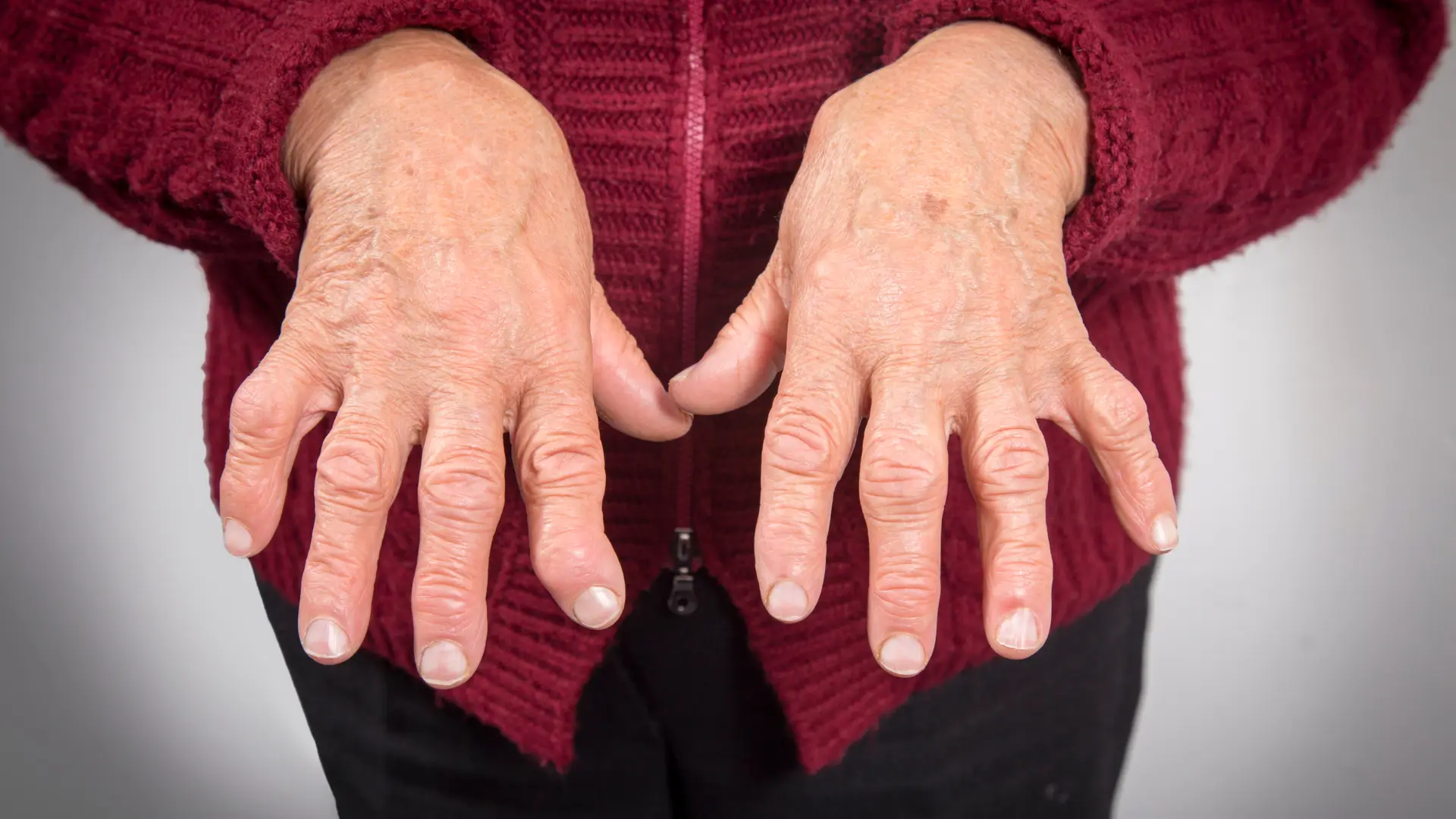
Actemra vs Orencia – Rheumatoid Arthritis Treatments Compared
Approximately 1.5 million Americans live with rheumatoid arthritis (RA), a chronic autoimmune condition characterized by joint inflammation and pain.

Actemra Indications – What Is It Used For?
Adhering to FDA-approved indications is essential for ensuring patient safety and maximizing treatment efficacy.
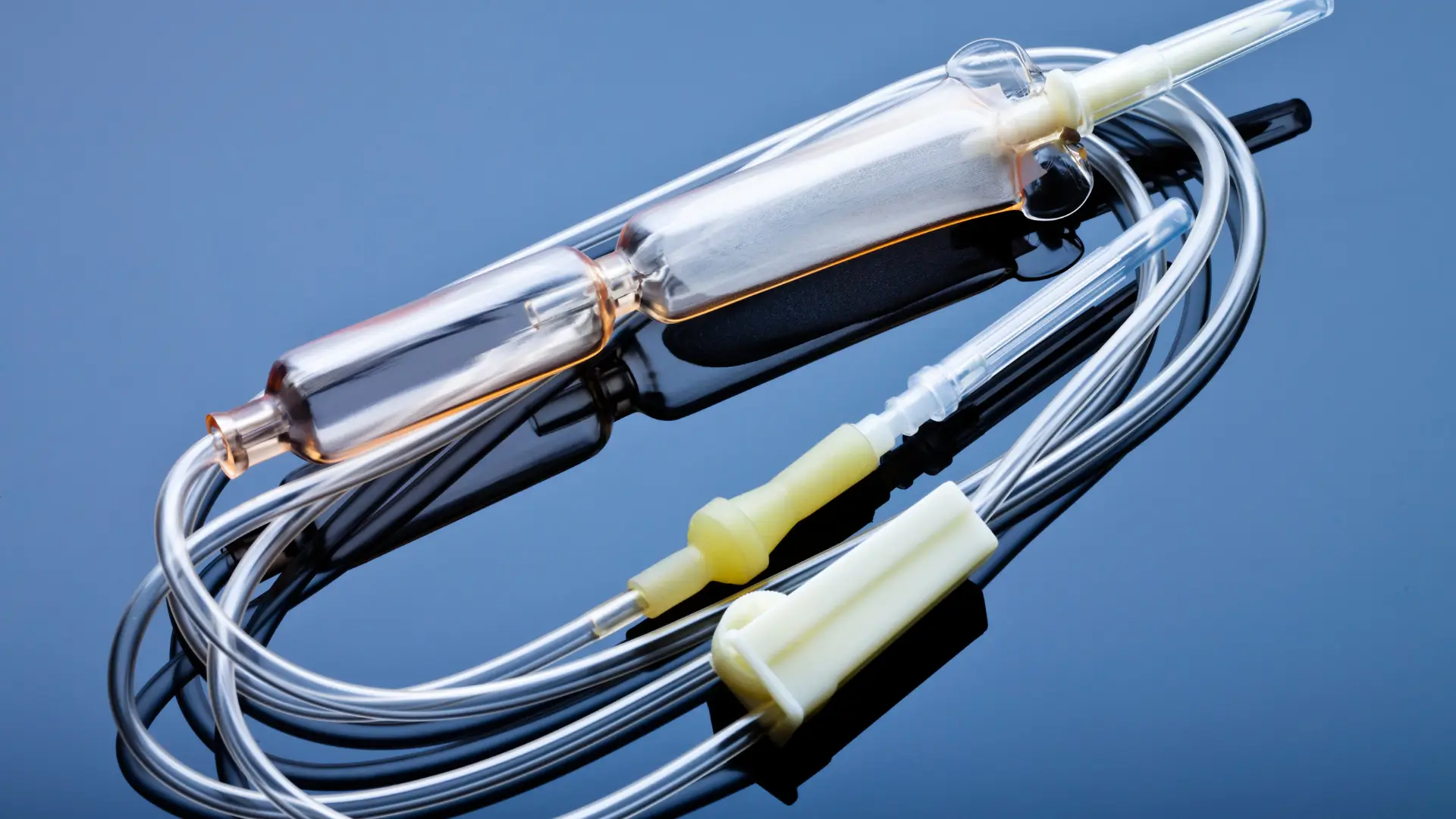
Actemra Infusion vs Injection – What’s the Difference?
A recent multinational study revealed that nearly 79% of patients with rheumatoid arthritis remained on tocilizumab therapy after six months—an impressive retention rate that highlights its enduring value in managing chronic inflammation.
Actemra Generic Name – About Tocilizumab
Tocilizumab, the generic name for Actemra, has garnered significant attention in the medical field due to its effectiveness in treating autoimmune disorders.
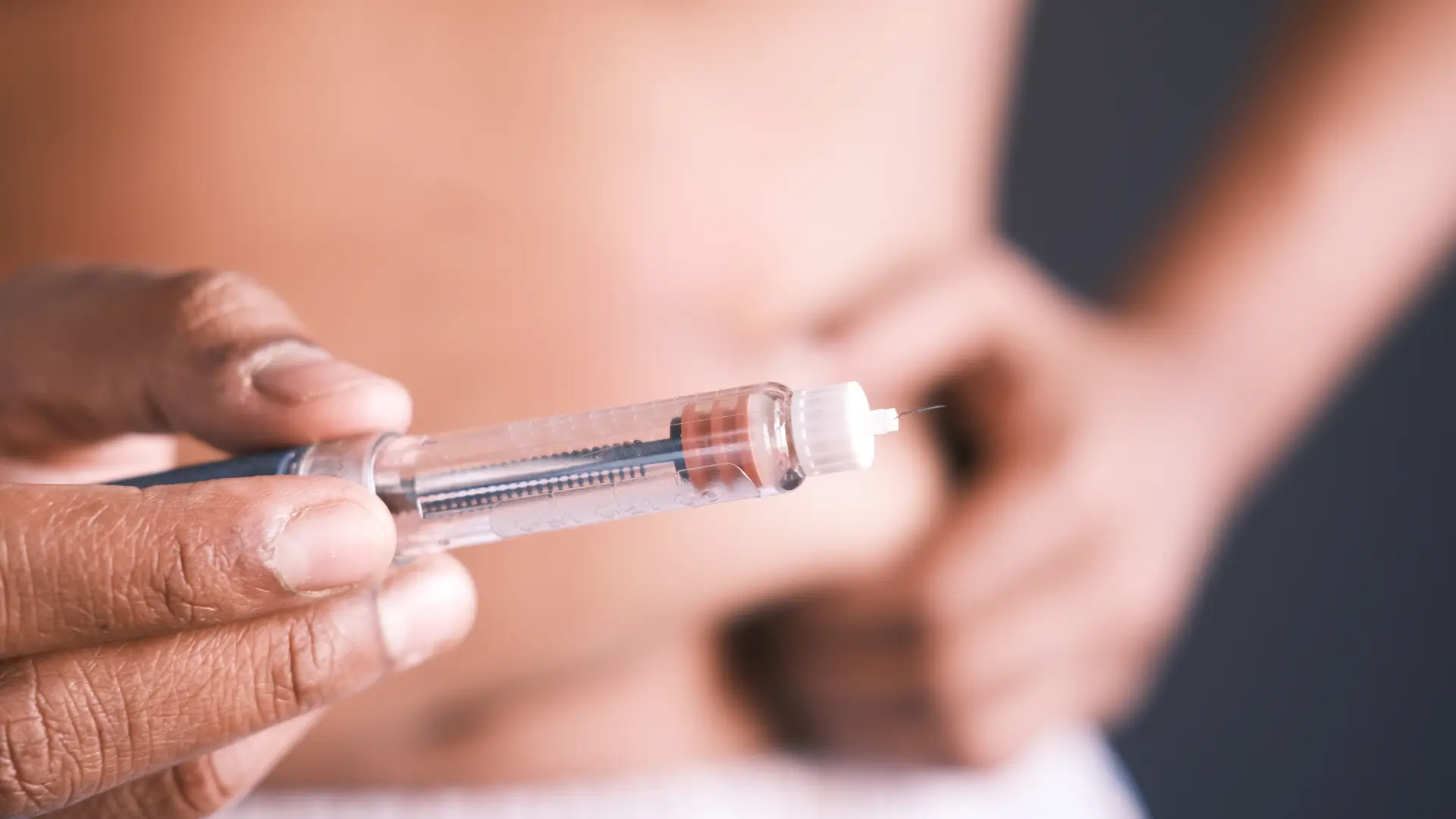
What Is Actemra? Is It a Biologic?
Biologic therapies have revolutionized the treatment of complex diseases, providing targeted solutions that can manage conditions like cancer, autoimmune disorders, and diabetes.
Actemra Dosing and Schedule
Recent clinical research published in BMC Medicine highlights a crucial aspect of non-invasive medical interventions—adhering to precise dosing and scheduling protocols.